Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment
- PMID: 36287933
- PMCID: PMC9607203
- DOI: 10.3390/toxins14100664
Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment
Abstract
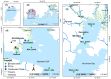
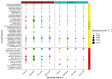
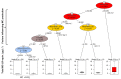
Africa’s water needs are often supported by eutrophic water bodies dominated by cyanobacteria posing health threats to riparian populations from cyanotoxins, and Lake Victoria is no exception. In two embayments of the lake (Murchison Bay and Napoleon Gulf), cyanobacterial surveys were conducted to characterize the dynamics of cyanotoxins in lake water and water treatment plants. Forty-six cyanobacterial taxa were recorded, and out of these, fourteen were considered potentially toxigenic (i.e., from the genera Dolichospermum, Microcystis, Oscillatoria, Pseudanabaena and Raphidiopsis). A higher concentration (ranging from 5 to 10 µg MC-LR equiv. L−1) of microcystins (MC) was detected in Murchison Bay compared to Napoleon Gulf, with a declining gradient from the inshore (max. 15 µg MC-LR equiv. L−1) to the open lake. In Murchison Bay, an increase in Microcystis sp. biovolume and MC was observed over the last two decades. Despite high cell densities of toxigenic Microcystis and high MC concentrations, the water treatment plant in Murchison Bay efficiently removed the cyanobacterial biomass, intracellular and dissolved MC to below the lifetime guideline value for exposure via drinking water (<1.0 µg MC-LR equiv. L−1). Thus, the potential health threats stem from the consumption of untreated water and recreational activities along the shores of the lake embayments. MC concentrations were predicted from Microcystis cell numbers regulated by environmental factors, such as solar radiation, wind speed in the N−S direction and turbidity. Thus, an early warning through microscopical counting of Microcystis cell numbers is proposed to better manage health risks from toxigenic cyanobacteria in Lake Victoria.
Keywords: Dolichospermum; Microcystis; drinking water; exposure routes; microcystins; rapid sand filtration; recreational areas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Paerl H.W., Gardner W.S., Havens K.E., Joyner A.R., McCarthy M.J., Newell S.E., Qin B., Scott J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae. 2016;54:213–222. doi: 10.1016/j.hal.2015.09.009. - DOI - PubMed
-
- Gray E., Elliott J.A., Mackay E.B., Folkard A.M., Keenan P.O., Jones I.D. Modelling lake cyanobacterial blooms: Disentangling the climate-driven impacts of changing mixed depth and water temperature. Freshw. Biol. 2019;64:2141–2155. doi: 10.1111/fwb.13402. - DOI
-
- Chorus I., Welker M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. Taylor & Francis; New York, NY, USA: 2021.
-
- Codd G.A., Meriluoto J., Metcalf J.S. Introduction: Cyanobacteria, Cyanotoxins, Their Human Impact, and Risk Management. In: Meriluoto J., Spoof L., Codd G.A., editors. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. 1st ed. John Wiley & Sons; London, UK: 2017. pp. 1–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

