Evaluation of a Novel Synthetic Peptide Derived from Cytolytic Mycotoxin Candidalysin
- PMID: 36287965
- PMCID: PMC9610734
- DOI: 10.3390/toxins14100696
Evaluation of a Novel Synthetic Peptide Derived from Cytolytic Mycotoxin Candidalysin
Abstract
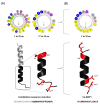
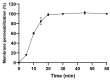
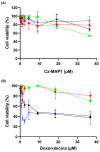
The importance of neuroinflammation in neurology is becoming increasingly apparent. In addition to neuroinflammatory diseases such as multiple sclerosis, the role of neuroinflammation has been identified in many non-inflammatory neurological disorders such as stroke, epilepsy, and cancer. The immune response within the brain involves the presence of CNS resident cells; mainly glial cells, such as microglia, the CNS resident macrophages. We evaluated the peptide Ca-MAP1 bioinspired on the C. albicans immature cytolytic toxin candidalysin to develop a less hemolytic peptide with anti-neuroinflammatory, antibacterial, and cytotoxic activity against tumor cells. In silico and in vitro studies were performed at various concentrations. Ca-MAP1 exhibits low hemolytic activity at lower concentrations and was not cytotoxic to MRC-5 and BV-2 cells. Ca-MAP1 showed activity against Acinetobacter baumannii, Escherichia coli ATCC, E. coli KPC, Klebsiella pneumoniae ATCC, Pseudomonas aeruginosa, and Staphylococcus aureus ATCC. Furthermore, Ca-MAP1 exhibits anti-neuroinflammatory activity in the BV-2 microglia model, with 93.78% inhibition of nitrate production at 18.1 µM. Ca-MAP1 presents cytotoxic activity against tumor cell line NCI-H292 at 36.3 μM, with an IC50 of 38.4 µM. Ca-MAP1 demonstrates results that qualify it to be evaluated in the next steps to promote the control of infections and provide an alternative antitumor therapy.
Keywords: Candida albicans; drug design; multiactivity peptide; neuroinflammation; peptide toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Liao D., Xiang D., Dang R., Xu P., Wang J., Han W., Fu Y., Yao D., Cao L., Jiang P. Neuroprotective Effects of Dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxid. Med. Cell. Longev. 2018;2018:9125601. doi: 10.1155/2018/9125601. - DOI - PMC - PubMed
-
- Boleti A.P.D.A., Frihling B.E.F., Silva P.S.E., Cardoso P.H.D.O., de Moraes L.F.R.N., Rodrigues T.A.A., Biembengute M.E.F., Koolen H.H.F., Migliolo L. Biochemical Aspects and Therapeutic Mechanisms of Cannabidiol in Epilepsy. Neurosci. Biobehav. Rev. 2022;132:1214–1228. doi: 10.1016/j.neubiorev.2020.09.027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

