Updating the Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Asia: A Systematic Review and Meta-Analysis
- PMID: 36288047
- PMCID: PMC9607256
- DOI: 10.3390/tropicalmed7100306
Updating the Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Asia: A Systematic Review and Meta-Analysis
Abstract
Background: Aedes aegypti and Aedes albopictus are two important vectors of several important arboviruses, including the dengue, chikungunya, and Zika viruses. Insecticide application is an important approach to reduce vector abundance during Aedes spp.-borne outbreaks in the absence of effective vaccines and treatments. However, insecticide overuse can result in the development of resistance, and careful monitoring of resistance markers is required.
Methods: This meta-analysis and systematic review explored the spatial and temporal patterns of insecticide resistance in Asia from 2000 to 2021. PubMed, Scopus, EbscoHost, and Embase were used to enhance the search capability. The random-effects model was applied for the 94 studies that met our inclusion criteria for qualitative synthesis and meta-analysis.
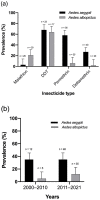
Results: Four major insecticides were studied (malathion, dichlorodiphenyltrichloroethane, permethrin, and deltamethrin). Dichlorodiphenyltrichloroethane resistance rates were high in both Ae. aegypti and Ae. albopictus (68% and 64%, respectively). Conversely, malathion resistance was less prevalent in Ae. aegypti (3%), and deltamethrin resistance was less common in Ae. albopictus (2%). Ae. aegypti displayed consistently high resistance rates (35%) throughout the study period, whereas the rate of insecticide resistance in Ae. albopictus increased from 5% to 12%. The rates of the major kdr mutations F1534C, V1016G, and S989P were 29%, 26%, and 22%, respectively.
Conclusions: Insecticide resistance in both Ae. aegypti and Ae. albopictus is widespread in Asia, although the rates vary by country. Continuous monitoring of the resistance markers and modification of the control strategies will be important for preventing unexpected outbreaks. This systematic review and meta-analysis provided up-to-date information on insecticide resistance in dengue-endemic countries in Asia.
Keywords: Aedes aegypti; Aedes albopictus; Asia; insecticide resistance; meta-analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- World Health Organization (WHO) 2022 Vector-Borne Diseases. [(accessed on 4 May 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

