Regulation of α-synuclein homeostasis and inflammasome activation by microglial autophagy
- PMID: 36288297
- PMCID: PMC9604594
- DOI: 10.1126/sciadv.abn1298
Regulation of α-synuclein homeostasis and inflammasome activation by microglial autophagy
Abstract
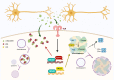
Autophagy clears protein aggregates, damaged cellular organelles, and pathogens through the lysosome. Although autophagy is highly conserved across all cell types, its activity in each cell is specifically adapted to carry out distinct physiological functions. The role of autophagy in neurons has been well characterized; however, in glial cells, its function remains largely unknown. Microglia are brain-resident macrophages that survey the brain to remove injured neurons, excessive synapses, protein aggregates, and infectious agents. Current studies have demonstrated that dysfunctional microglia contribute to neurodegenerative diseases. In Alzheimer's disease animal models, microglia play a critical role in regulating amyloid plaque formation and neurotoxicity. However, how microglia are involved in Parkinson's disease (PD) remains poorly understood. Propagation of aggregated α-synuclein via cell-to-cell transmission and neuroinflammation have emerged as important mechanisms underlying neuropathologies in PD. Here, we review converging evidence that microglial autophagy maintains α-synuclein homeostasis, regulates neuroinflammation, and confers neuroprotection in PD experimental models.
Figures
References
-
- Dikic I., Elazar Z., Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018). - PubMed
-
- Kirkin V., Rogov V. V., A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76, 268–285 (2019). - PubMed
-
- Mizushima N., Autophagy: Process and function. Genes Dev. 21, 2861–2873 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

