Rapid and precise measurement of carbonate clumped isotopes using laser spectroscopy
- PMID: 36288314
- PMCID: PMC12419128
- DOI: 10.1126/sciadv.abq0611
Rapid and precise measurement of carbonate clumped isotopes using laser spectroscopy
Abstract
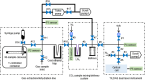
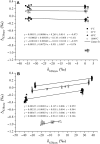
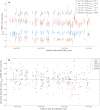
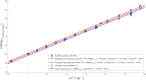
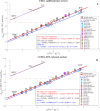
Carbonate clumped isotope abundance is an important paleothermometer, but measurement is difficult, slow, and subject to cardinal mass (m/z) interferences using isotope ratio mass spectrometry (IRMS). Here, we describe an optical spectroscopic measurement of carbonate clumped isotopes. We have adapted a tunable infrared laser differential absorption spectrometer (TILDAS) system to measure the abundances of four CO2 isotopologues used for clumped isotope thermometry. TILDAS achieves the same precision (0.01‰ SE) as IRMS measurements rapidly (∼50 min per carbonate analysis) and using small samples (<2 mg of calcite), without making assumptions about 17O abundance in the sample. A temperature calibration based on 406 analyses of CO2 produced by digestion of 51 synthetic carbonates equilibrated at 6° to 1100°C is consistent with results for natural carbonates and previous calibrations. Our system results were indistinguishable from IRMS systems after replicating the InterCarb interlaboratory calibration. Measurement by TILDAS could change the landscape for clumped isotope analysis.
Figures

References
-
- Wang Z., Schauble E. A., Eiler J. M., Equilibrium thermodynamics of multiply substituted isotopologues of molecular gases. Geochim. Cosmochim. Acta 68, 4779–4797 (2004).
-
- Schauble E. A., Ghosh P., Eiler J. M., Preferential formation of 13C-18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochim. Cosmochim. Acta 70, 2510–2529 (2006).
-
- Ghosh P., Adkins J., Affek H., Balta B., Guo W., Schauble E. A., Schrag D., Eiler J. M., 13C–18O bonds in carbonate minerals: A new kind of paleothermometer. Geochim. Cosmochim. Acta 70, 1439–1456 (2006).
-
- Dennis K. J., Affek H. P., Passey B. H., Schrag D. P., Eiler J. M., Defining an absolute reference frame for “clumped” isotope studies of CO2. Geochim. Cosmochim. Acta 75, 7117–7131 (2011).
-
- Bernasconi S. M., Daëron M., Bergmann K. D., Bonifacie M., Meckler A. N., Affek H. P., Anderson N., Bajnai D., Barkan E., Beverly E., Blamart D., Burgener L., Calmels D., Chaduteau C., Clog M., Davidheiser-Kroll B., Davies A., Dux F., Eiler J., Elliott B., Fetrow A. C., Fiebig J., Goldberg S., Hermoso M., Huntington K. W., Hyland E., Ingalls M., Jaggi M., John C. M., Jost A. B., Katz S., Kelson J., Kluge T., Kocken I. J., Laskar A., Leutert T. J., Liang D., Lucarelli J., Mackey T. J., Mangenot X., Meinicke N., Modestou S. E., Müller I. A., Murray S., Neary A., Packard N., Passey B. H., Pelletier E., Petersen S., Piasecki A., Schauer A., Snell K. E., Swart P. K., Tripati A., Upadhyay D., Vennemann T., Winkelstern I., Yarian D., Yoshida N., Zhang N., Ziegler M., InterCarb: A community effort to improve interlaboratory standardization of the carbonate clumped isotope thermometer using carbonate standards. Geochem. Geophys. Geosyst. 22, e2020GC009588 (2021). - PMC - PubMed
LinkOut - more resources
Full Text Sources

