Alliance A071401: Phase II Trial of Focal Adhesion Kinase Inhibition in Meningiomas With Somatic NF2 Mutations
- PMID: 36288512
- PMCID: PMC9870228
- DOI: 10.1200/JCO.21.02371
Alliance A071401: Phase II Trial of Focal Adhesion Kinase Inhibition in Meningiomas With Somatic NF2 Mutations
Abstract
Purpose: Patients with progressive or recurrent meningiomas have limited systemic therapy options. Focal adhesion kinase (FAK) inhibition has a synthetic lethal relationship with NF2 loss. Given the predominance of NF2 mutations in meningiomas, we evaluated the efficacy of GSK2256098, a FAK inhibitor, as part of the first genomically driven phase II study in recurrent or progressive grade 1-3 meningiomas.
Patients and methods: Eligible patients whose tumors screened positively for NF2 mutations were treated with GSK2256098, 750 mg orally twice daily, until progressive disease. Efficacy was evaluated using two coprimary end points: progression-free survival at 6 months (PFS6) and response rate by Macdonald criteria, where PFS6 was evaluated separately within grade-based subgroups: grade 1 versus 2/3 meningiomas. Per study design, the FAK inhibitor would be considered promising in this patient population if either end point met the corresponding decision criteria for efficacy.
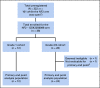
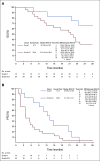
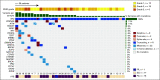
Results: Of 322 patients screened for all mutation cohorts of the study, 36 eligible and evaluable patients with NF2 mutations were enrolled and treated: 12 grade 1 and 24 grade 2/3 patients. Across all grades, one patient had a partial response and 24 had stable disease as their best response to treatment. In grade 1 patients, the observed PFS6 rate was 83% (10/12 patients; 95% CI, 52 to 98). In grade 2/3 patients, the observed PFS6 rate was 33% (8/24 patients; 95% CI, 16 to 55). The study met the PFS6 efficacy end point both for the grade 1 and the grade 2/3 cohorts. Treatment was well tolerated; seven patients had a maximum grade 3 adverse event that was at least possibly related to treatment with no grade 4 or 5 events.
Conclusion: GSK2256098 was well tolerated and resulted in an improved PFS6 rate in patients with recurrent or progressive NF2-mutated meningiomas, compared with historical controls. The criteria for promising activity were met, and FAK inhibition warrants further evaluation for this patient population.
Trial registration: ClinicalTrials.gov NCT02523014.
Conflict of interest statement
Alliance A071401: Phase II Trial of Focal Adhesion Kinase Inhibition in Meningiomas With Somatic NF2 Mutations
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233320/CA/NCI NIH HHS/United States
- R37 CA225655/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233324/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA233193/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

