Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer
- PMID: 36288547
- PMCID: PMC9928626
- DOI: 10.1200/JCO.22.00739
Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer
Abstract
Purpose: Overexpression of c-Met protein and epidermal growth factor receptor (EGFR) mutations can co-occur in non-small-cell lung cancer (NSCLC), providing strong rationale for dual targeting. Telisotuzumab vedotin (Teliso-V), a first-in-class antibody-drug conjugate targeting c-Met, has shown a tolerable safety profile and antitumor activity as monotherapy. Herein, we report the results of a phase Ib study (ClinicalTrials.gov identifier: NCT02099058) evaluating Teliso-V plus erlotinib, an EGFR tyrosine kinase inhibitor (TKI), in patients with c-Met-positive (+) NSCLC.
Patients and methods: This study evaluated Teliso-V (2.7 mg/kg once every 21 days) plus erlotinib (150 mg once daily) in adult patients (age ≥ 18 years) with c-Met+ NSCLC. Later enrollment required presence of an EGFR-activating mutation (EGFR-M+) and progression on a prior EGFR TKI. End points included safety, pharmacokinetics, objective response rate (ORR), and progression-free survival (PFS). The efficacy-evaluable population consisted of c-Met+ patients (confirmed histology [H]-score ≥ 150) who had at least one postbaseline scan; c-Met+ patients with H-scores ≥ 225 were classified as c-Met high.
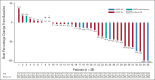
Results: As of January 2020, 42 patients were enrolled (N = 36 efficacy-evaluable). Neuropathies were the most common any-grade adverse events reported, with 24 of 42 patients (57%) experiencing at least one event. The pharmacokinetic profile of Teliso-V plus erlotinib was similar to Teliso-V monotherapy. Median PFS for all efficacy-evaluable patients was 5.9 months (95% CI, 2.8 to not reached). ORR for EGFR-M+ patients (n = 28) was 32.1%. Of EGFR-M+ patients, those who were c-Met high (n = 15) had an ORR of 52.6%. Median PFS was 6.8 months for non-T790M+ and for those whose T790M status was unknown, versus 3.7 months for T790M+.
Conclusion: Teliso-V plus erlotinib showed encouraging antitumor activity and acceptable toxicity in EGFR TKI-pretreated patients with EGFR-M+, c-Met+ NSCLC.
Figures

Comment in
-
Telisotuzumab vedotin with erlotinib in the treatment of non-small cell lung cancer: a well MET combination?Transl Lung Cancer Res. 2023 Aug 30;12(8):1826-1829. doi: 10.21037/tlcr-23-288. Epub 2023 Jul 28. Transl Lung Cancer Res. 2023. PMID: 37691875 Free PMC article. No abstract available.
References
-
- Ferlay J, Ervik M, Lam F, et al. (eds): Cancer Today (Powered by GLOBOCAN 2020). Lyon, International Agency for Research on Cancer, 2020. https://gco.iarc.fr/today
-
- Howlander N, Noone AM, Krapcho M, et al. : SEER Cancer Statistics Review. Bethesda, MD, National Cancer Institute, 2020, pp 1975-2017. https://seer.cancer.gov/csr/1975_2017
-
- Cooper CS, Park M, Blair DG, et al. : Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311:29-33, 1984 - PubMed
-
- Bottaro DP, Rubin JS, Faletto DL, et al. : Identification of the hepatocyte growth factor receptor as the c-Met proto-oncogene product. Science 251:802-804, 1991 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

