Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial
- PMID: 36288823
- PMCID: PMC9615980
- DOI: 10.1136/rmdopen-2022-002686
Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial
Abstract
Objective: Sodium-glucose cotransporter-2 inhibitors have been identified profound renal/cardiac protective effects in different diseases. Here, we assessed the safety and efficacy of dapagliflozin among adult patients with systemic lupus erythematosus (SLE).
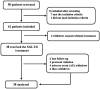
Methods: We conducted a single-arm, open-label, investigator-initiated phase I/II trial of dapagliflozin in Chinese patients with SLE with/without lupus nephritis (LN). Patients received oral dapagliflozin at a daily dose of 10 mg added to the standard of care for 6 months. The primary end point was the safety profile. The secondary efficacy end points were composite assessments of disease activity.
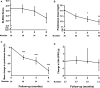
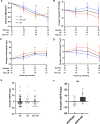
Results: A total of 38 eligible patients were enrolled. Overall, 19 (50%) adverse events (AEs) were recorded, including 8 (21%) AEs leading to drug discontinuation, of which 4 (10.5%) were attributed to dapagliflozin. Two serious AEs (one of major lupus flare and one of fungal pneumonia) were recorded. Lower urinary tract infection was observed in one (2.63%) patient. The secondary end points revealed no improvement of SLE Disease Activity Index scores or proteinuria (among 17 patients with LN); the cumulative lupus flare rate was 18%, and a reduction of ~30% in the prednisone dose was captured. Net changes in body mass index (-0.50 kg/m2), systolic blood pressure (-3.94 mm Hg) and haemoglobin levels (+8.26 g/L) were detected. The overall estimated glomerular filtration rate (eGFR) was stable, and there was an improvement in the eGFR slope among patients with LN with a baseline eGFR <90 mL/min/1.73 m2.
Conclusion: Dapagliflozin had an acceptable safety profile in adult patients with SLE. Its possible renal/cardiac protective effects and long-term safety issues in patients with SLE, patients with LN in particular, call for further exploration.
Trial registration number: ChiCTR1800015030.
Keywords: lupus erythematosus, systemic; lupus nephritis; outcome and process assessment, health care.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
