Revealing the role of ionic liquids in promoting fuel cell catalysts reactivity and durability
- PMID: 36289200
- PMCID: PMC9606256
- DOI: 10.1038/s41467-022-33895-5
Revealing the role of ionic liquids in promoting fuel cell catalysts reactivity and durability
Abstract
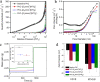
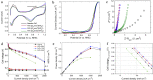
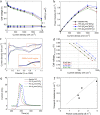
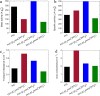
Ionic liquids (ILs) have shown to be promising additives to the catalyst layer to enhance oxygen reduction reaction in polymer electrolyte fuel cells. However, fundamental understanding of their role in complex catalyst layers in practically relevant membrane electrode assembly environment is needed for rational design of highly durable and active platinum-based catalysts. Here we explore three imidazolium-derived ionic liquids, selected for their high proton conductivity and oxygen solubility, and incorporate them into high surface area carbon black support. Further, we establish a correlation between the physical properties and electrochemical performance of the ionic liquid-modified catalysts by providing direct evidence of ionic liquids role in altering hydrophilic/hydrophobic interactions within the catalyst layer interface. The resulting catalyst with optimized interface design achieved a high mass activity of 347 A g-1Pt at 0.9 V under H2/O2, power density of 0.909 W cm-2 under H2/air and 1.5 bar, and had only 0.11 V potential decrease at 0.8 A cm-2 after 30 k accelerated stress test cycles. This performance stems from substantial enhancement in Pt utilization, which is buried inside the mesopores and is now accessible due to ILs addition.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ejigu, A. & Walsh, D. A. Electrocatalysis in room temperature ionic liquids BT. in Electrochemistry in Ionic Liquids: Vol. 2: Applications (ed. Torriero, A. A. J.) 483–506 (Springer International Publishing, 2015)
-
- Dumont JH, et al. Nitrogen-doped graphene oxide electrocatalysts for the oxygen reduction reaction. ACS Appl. Nano Mater. 2019;2:1675–1682.
-
- Kabir S, Artyushkova K, Serov A, Atanassov P. Role of nitrogen moieties in N-doped 3D-graphene nanosheets for oxygen electroreduction in acidic and alkaline media. ACS Appl. Mater. Interfaces. 2018;10:11623–11632. - PubMed
-
- Shao Y, Sui J, Yin G, Gao Y. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2008;79:89–99.
-
- Ding D, et al. Plasma-assisted nitrogen doping of graphene-encapsulated Pt nanocrystals as efficient fuel cell catalysts. J. Mater. Chem. A. 2014;2:472–477.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

