Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia
- PMID: 36289368
- PMCID: PMC10805109
- DOI: 10.1038/s41574-022-00757-5
Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia
Abstract
Although promising therapeutics are in the pipeline, bariatric surgery (also known as metabolic surgery) remains our most effective strategy for the treatment of obesity and type 2 diabetes mellitus (T2DM). Of the many available options, Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are currently the most widely used procedures. RYGB and VSG have very different anatomical restructuring but both surgeries are effective, to varying degrees, at inducing weight loss and T2DM remission. Both weight loss-dependent and weight loss-independent alterations in multiple tissues (such as the intestine, liver, pancreas, adipose tissue and skeletal muscle) yield net improvements in insulin resistance, insulin secretion and insulin-independent glucose metabolism. In a subset of patients, post-bariatric hypoglycaemia can develop months to years after surgery, potentially reflecting the extreme effects of potent glucose reduction after surgery. This Review addresses the effects of bariatric surgery on glucose regulation and the potential mechanisms responsible for both the resolution of T2DM and the induction of hypoglycaemia.
© 2022. Springer Nature Limited.
Figures
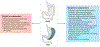


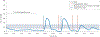

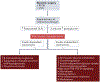
References
-
-
Schauer PR et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N. Engl. J. Med 376, 641–651 (2017).
This 5-year follow-up to the STAMPEDE clinical trial randomized patients with T2DM to RYGB, VSG or medical management, and showed that RYGB and VSG were superior to medical therapy in terms of weight loss, glycaemic control and reduction in medication use.
-
-
- Mingrone G. et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 397, 293–304 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

