Redox-Regulation in Cancer Stem Cells
- PMID: 36289675
- PMCID: PMC9598867
- DOI: 10.3390/biomedicines10102413
Redox-Regulation in Cancer Stem Cells
Abstract
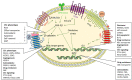
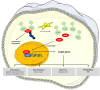
Cancer stem cells (CSCs) represent a small subset of slowly dividing cells with tumor-initiating ability. They can self-renew and differentiate into all the distinct cell populations within a tumor. CSCs are naturally resistant to chemotherapy or radiotherapy. CSCs, thus, can repopulate a tumor after therapy and are responsible for recurrence of disease. Stemness manifests itself through, among other things, the expression of stem cell markers, the ability to induce sphere formation and tumor growth in vivo, and resistance to chemotherapeutics and irradiation. Stemness is maintained by keeping levels of reactive oxygen species (ROS) low, which is achieved by enhanced activity of antioxidant pathways. Here, cellular sources of ROS, antioxidant pathways employed by CSCs, and underlying mechanisms to overcome resistance are discussed.
Keywords: CD13; Nrf2-sinaling; ROS; Sonic Hedgehog signaling; Wnt-signaling; antioxidant signaling pathways; cancer stem cell; drug resistance.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

