Recent Advances in the Development of Anti-FLT3 CAR T-Cell Therapies for Treatment of AML
- PMID: 36289703
- PMCID: PMC9598885
- DOI: 10.3390/biomedicines10102441
Recent Advances in the Development of Anti-FLT3 CAR T-Cell Therapies for Treatment of AML
Abstract
Following the success of the anti-CD19 chimeric antigen receptor (CAR) T-cell therapies against B-cell malignancies, the CAR T-cell approach is being developed towards other malignancies like acute myeloid leukemia (AML). Treatment options for relapsed AML patients are limited, and the upregulation of the FMS-like tyrosine kinase 3 (FLT3) in malignant T-cells is currently not only being investigated as a prognostic factor, but also as a target for new treatment options. In this review, we provide an overview and discuss different approaches of current anti-FLT3 CAR T-cells under development. In general, these therapies are effective both in vitro and in vivo, however the safety profile still needs to be further investigated. The first clinical trials have been initiated, and the community now awaits clinical evaluation of the approach of targeting FLT3 with CAR T-cells.
Keywords: AML; CAR; CAR T; FLT3; FLT3L; FMS-like tyrosine kinase-3; chimeric antigen receptor; leukemia.
Conflict of interest statement
The authors declare the following competing interests: ROB holds equity in Graphite Bio and UNIKUM Tx. ROB is a part-time employee in UNIKUM Tx. None of the companies were involved in the present study. The remaining authors declare no competing interests.
Figures

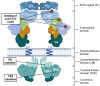
References
-
- Locke F.L., Neelapu S.S., Bartlett N.L., Siddiqi T., Chavez J.C., Hosing C.M., Ghobadi A., Budde L.E., Bot A., Rossi J.M., et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol. Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. - DOI - PMC - PubMed
-
- CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. [(accessed on 15 June 2022)];2022 June; Available online: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells.
Publication types
Grants and funding
- 4-1612-391/1/Danish health authorities (SST)
- NNF19OC0058238/Novo Nordisk Foundation
- NNF21OC0071259/Novo Nordisk Foundation
- 8056-00010B/Innovation Fund Denmark
- CF20-0424/Carlsberg Foundation
- CF17-0129/Carlsberg Foundation
- R238-2016-3349/Lundbeck Foundation
- R303-2018-3571/Lundbeck Foundation
- 101041231/ERC Starting Grant
- 101057438/Horizon Research and Innovation Actions
- 0134-00113B/Independent Research Fund Denmark
- 0242-00009B/Independent Research Fund Denmark
- 9144-00001B/Independent Research Fund Denmark
LinkOut - more resources
Full Text Sources
Miscellaneous

