Neuroprotective Effects of gH625-lipoPACAP in an In Vitro Fluid Dynamic Model of Parkinson's Disease
- PMID: 36289905
- PMCID: PMC9599564
- DOI: 10.3390/biomedicines10102644
Neuroprotective Effects of gH625-lipoPACAP in an In Vitro Fluid Dynamic Model of Parkinson's Disease
Abstract
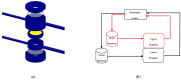
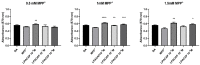
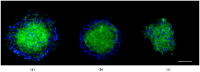
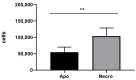
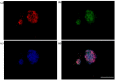
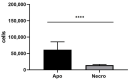
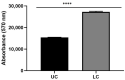
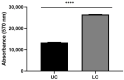
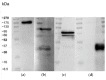
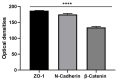
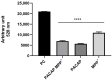
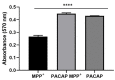
Parkinson's disease (PD) is an aggressive and devastating age-related disorder. Although the causes are still unclear, several factors, including genetic and environmental, are involved. Except for symptomatic drugs, there are not, to date, any real cures for PD. For this purpose, it is necessary develop a model to better study this disease. Neuroblastoma cell line, SH-SY5Y, differentiated with retinoic acid represents a good in vitro model to explore PD, since it maintains growth cells to differentiated neurons. In the present study, SH-SY5Y cells were treated with 1-methyl-4-phenylpyridinium (MPP+), a neurotoxin that induces Parkinsonism, and the neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP), delivered by functionalized liposomes in a blood-brain barrier fluid dynamic model, were evaluated. We demonstrated PACAP neuroprotective effects when delivered by gH625-liposome on MPP+-damaged SH-SY5Y spheroids.
Keywords: PACAP; Parkinson’s disease; millifluidic model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
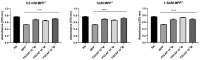

Similar articles
-
Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson's disease model.Neuropharmacology. 2016 Sep;108:440-50. doi: 10.1016/j.neuropharm.2015.05.014. Epub 2015 May 22. Neuropharmacology. 2016. PMID: 26006268
-
gH625-liposomes deliver PACAP through a dynamic in vitro model of the blood-brain barrier.Front Physiol. 2022 Aug 19;13:932099. doi: 10.3389/fphys.2022.932099. eCollection 2022. Front Physiol. 2022. PMID: 36060696 Free PMC article.
-
gH625-liposomes as tool for pituitary adenylate cyclase-activating polypeptide brain delivery.Sci Rep. 2019 Jun 24;9(1):9183. doi: 10.1038/s41598-019-45137-8. Sci Rep. 2019. PMID: 31235716 Free PMC article.
-
SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease.Chin Med J (Engl). 2010 Apr 20;123(8):1086-92. Chin Med J (Engl). 2010. PMID: 20497720 Review.
-
Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide.Curr Pharm Des. 2004;10(23):2861-89. doi: 10.2174/1381612043383548. Curr Pharm Des. 2004. PMID: 15379674 Review.
Cited by
-
Tuning Peptide-Based Nanofibers for Achieving Selective Doxorubicin Delivery in Triple-Negative Breast Cancer.Int J Nanomedicine. 2024 Jun 18;19:6057-6084. doi: 10.2147/IJN.S453958. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38911501 Free PMC article.
-
Study on the Pharmacological Efficacy and Mechanism of Dual-Target Liposome Complex AD808 Against Alzheimer's Disease.Pharmaceuticals (Basel). 2025 Jun 29;18(7):977. doi: 10.3390/ph18070977. Pharmaceuticals (Basel). 2025. PMID: 40732267 Free PMC article.
-
An Overview of Supramolecular Platforms Boosting Drug Delivery.Bioinorg Chem Appl. 2023 Nov 13;2023:8608428. doi: 10.1155/2023/8608428. eCollection 2023. Bioinorg Chem Appl. 2023. PMID: 38028018 Free PMC article. Review.
-
Utilization of nanotechnology to surmount the blood-brain barrier in disorders of the central nervous system.Mater Today Bio. 2025 Jan 4;31:101457. doi: 10.1016/j.mtbio.2025.101457. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39896289 Free PMC article. Review.
-
More Than Three Decades After Discovery of the Neuroprotective Effect of PACAP, What Is Still Preventing Its Clinical Use?J Mol Neurosci. 2025 Jun 21;75(3):80. doi: 10.1007/s12031-025-02366-z. J Mol Neurosci. 2025. PMID: 40542938 Free PMC article. Review.
References
-
- Lo Bianco C., Schneider B.L., Bauer M., Sajadi A., Brice A., Iwatsubo T., Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an asynuclein rat model of Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

