Injectable Crosslinked Genipin Hybrid Gelatin-PVA Hydrogels for Future Use as Bioinks in Expediting Cutaneous Healing Capacity: Physicochemical Characterisation and Cytotoxicity Evaluation
- PMID: 36289912
- PMCID: PMC9599713
- DOI: 10.3390/biomedicines10102651
Injectable Crosslinked Genipin Hybrid Gelatin-PVA Hydrogels for Future Use as Bioinks in Expediting Cutaneous Healing Capacity: Physicochemical Characterisation and Cytotoxicity Evaluation
Abstract
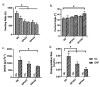
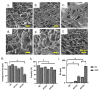
The irregular shape and depth of wounds could be the major hurdles in wound healing for the common three-dimensional foam, sheet, or film treatment design. The injectable hydrogel is a splendid alternate technique to enhance healing efficiency post-implantation via injectable or 3D-bioprinting technologies. The authentic combination of natural and synthetic polymers could potentially enhance the injectability and biocompatibility properties. Thus, the purpose of this study was to characterise a hybrid gelatin−PVA hydrogel crosslinked with genipin (GNP; natural crosslinker). In brief, gelatin (GE) and PVA were prepared in various concentrations (w/v): GE, GPVA3 (3% PVA), and GPVA5 (5% PVA), followed by a 0.1% (w/v) genipin (GNP) crosslink, to achieve polymerisation in three minutes. The physicochemical and biocompatibility properties were further evaluated. GPVA3_GNP and GPVA5_GNP with GNP demonstrated excellent physicochemical properties compared to GE_GNP and non-crosslinked hydrogels. GPVA5_GNP significantly displayed the optimum swelling ratio (621.1 ± 93.18%) and excellent hydrophilicity (38.51 ± 2.58°). In addition, GPVA5_GNP showed an optimum biodegradation rate (0.02 ± 0.005 mg/h) and the highest mechanical strength with the highest compression modulus (2.14 ± 0.06 MPa). In addition, the surface and cross-sectional view for scanning electron microscopy (SEM) displayed that all of the GPVA hydrogels have optimum average pore sizes (100−199 μm) with interconnected pores. There were no substantial changes in chemical analysis, including FTIR, XRD, and EDX, after PVA and GNP intervention. Furthermore, GPVA hydrogels influenced the cell biocompatibility, which successfully indicated >85% of cell viability. In conclusion, gelatin−PVA hydrogels crosslinked with GNP were proven to have excellent physicochemical, mechanical, and biocompatibility properties, as required for potential bioinks for chronic wound healing.
Keywords: 3D-bioprinting; PVA; bioinks; gelatin; injectable hydrogel; skin tissue; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Performance of hybrid gelatin-PVA bioinks integrated with genipin through extrusion-based 3D bioprinting: An in vitro evaluation using human dermal fibroblasts.Int J Bioprint. 2023 Feb 7;9(3):677. doi: 10.18063/ijb.677. eCollection 2023. Int J Bioprint. 2023. PMID: 37274005 Free PMC article.
-
Multifunctionalised skin substitute of hybrid gelatin-polyvinyl alcohol bioinks for chronic wound: injectable vs. 3D bioprinting.Drug Deliv Transl Res. 2024 Apr;14(4):1005-1027. doi: 10.1007/s13346-023-01447-z. Epub 2023 Nov 8. Drug Deliv Transl Res. 2024. PMID: 37938542
-
Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models.Pharmaceuticals (Basel). 2022 Oct 27;15(11):1328. doi: 10.3390/ph15111328. Pharmaceuticals (Basel). 2022. PMID: 36355501 Free PMC article.
-
Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair.Tissue Eng Part A. 2021 Jun;27(11-12):679-702. doi: 10.1089/ten.TEA.2020.0350. Epub 2021 Mar 9. Tissue Eng Part A. 2021. PMID: 33499750 Review.
-
Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings.Polymers (Basel). 2021 Aug 31;13(17):2959. doi: 10.3390/polym13172959. Polymers (Basel). 2021. PMID: 34502997 Free PMC article. Review.
Cited by
-
A Comprehensive Review of Honey-Containing Hydrogel for Wound Healing Applications.Gels. 2025 Mar 12;11(3):194. doi: 10.3390/gels11030194. Gels. 2025. PMID: 40136899 Free PMC article. Review.
-
Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing.Gels. 2023 Sep 14;9(9):748. doi: 10.3390/gels9090748. Gels. 2023. PMID: 37754429 Free PMC article.
-
Development of Biocompatible Coatings with PVA/Gelatin Hydrogel Films on Vancomycin-Loaded Titania Nanotubes for Controllable Drug Release.ACS Omega. 2024 Aug 22;9(35):37052-37062. doi: 10.1021/acsomega.4c03942. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246498 Free PMC article.
-
Characterization of Dual-Layer Hybrid Biomatrix for Future Use in Cutaneous Wound Healing.Materials (Basel). 2023 Jan 29;16(3):1162. doi: 10.3390/ma16031162. Materials (Basel). 2023. PMID: 36770168 Free PMC article.
-
Injectable Gelatin-Palmitoyl-GDPH Hydrogels as Bioinks for Future Cutaneous Regeneration: Physicochemical Characterization and Cytotoxicity Assessment.Polymers (Basel). 2024 Dec 27;17(1):41. doi: 10.3390/polym17010041. Polymers (Basel). 2024. PMID: 39795444 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

