Anti-Tuberculosis Activity of Three Carbapenems, Clofazimine and Nitazoxanide Using a Novel Ex Vivo Phenotypic Drug Susceptibility Model of Human Tuberculosis
- PMID: 36289932
- PMCID: PMC9598577
- DOI: 10.3390/antibiotics11101274
Anti-Tuberculosis Activity of Three Carbapenems, Clofazimine and Nitazoxanide Using a Novel Ex Vivo Phenotypic Drug Susceptibility Model of Human Tuberculosis
Abstract
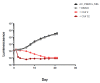
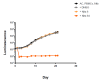
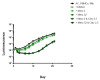
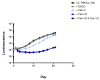
We evaluated a novel physiological 3-D bioelectrospray model of the tuberculosis (TB) granuloma to test the activity of a known anti-TB drug, clofazimine; three carbapenems with potential activity, including one currently used in therapy; and nitazoxanide, an anti-parasitic compound with possible TB activity (all chosen as conventional drug susceptibility was problematical). PBMCs collected from healthy donors were isolated and infected with M. tuberculosis H37Rv lux (i.e., luciferase). Microspheres were generated with the infected cells; the anti-microbial compounds were added and bacterial luminescence was monitored for at least 21 days. Clavulanate was added to each carbapenem to inhibit beta-lactamases. M. tuberculosis (MTB) killing efficacy was dose dependent. Clofazimine was the most effective drug inhibiting MTB growth at 2 mg/L with good killing activity at both concentrations tested. It was the only drug that killed bacteria at the lowest concentration tested. Carbapenems showed modest initial activity that was lost at around day 10 of incubation and clavulanate did not increase killing activity. Of the carbapenems tested, tebipenem was the most efficient in killing MTB, albeit at a high concentration. Nitazoxanide was effective only at concentrations not achievable with current dosing (although this might partly have been an artefact related to extensive protein binding).
Keywords: anti-microbial drug resistance; drug susceptibility testing; three-dimensional bioelectrospray; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- World Health Organization . Global Tuberculosis Report 2019. World Health Organization; Geneva, Switzerland: 2019.
-
- Tezera L.T., Bielecka M.K., Chancellor A., Reichmann M.T., Basim A.l., Shammari B.A., Brace P., Batty A., Tocheva A., Jogai S., et al. Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model. eLife. 2017;6:e21283. doi: 10.7554/eLife.21283. - DOI - PMC - PubMed
-
- Bielecka M.K., Tezera L.B., Zmijan R., Drobniewski F., Zhang X., Jayasinghe S., Elkington P. A Bioengineered Three Dimensional Cell Culture Platform Integrated with Microfluidics To Address Antimicrobial Resistance in Tuberculosis. mBio. 2017;8:e02073-16. doi: 10.1128/mBio.02073-16. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

