Antimicrobial Activity of Lactones
- PMID: 36289985
- PMCID: PMC9598898
- DOI: 10.3390/antibiotics11101327
Antimicrobial Activity of Lactones
Abstract
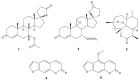
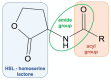
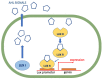
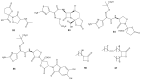
The development of bacterial resistance to antibiotics and the consequent lack of effective therapy is one of the biggest problems in modern medicine. A consequence of these processes is an urgent need to continuously design and develop novel antimicrobial agents. Among the compounds showing antimicrobial potential, lactones are a group to explore. For centuries, their antimicrobial activities have been used in folk medicine. Currently, novel lactone compounds are continuously described in the literature. Some of those structures exhibit high antimicrobial potential and some are an inspiration for design and synthesis of future drugs. This paper describes recent developments on antimicrobial lactones with smaller ring sizes, up to seven membered ε-lactones. Their isolation from natural sources, chemical synthesis, synergistic activity with antibiotics, and effects on quorum sensing are presented herein.
Keywords: antibacterial activity; antimicrobial compounds; isolation of lactones; lactones; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



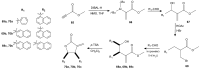



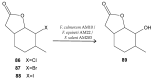

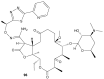
References
-
- Sartori S.K., Diaz M.A.N., Diaz-Muñoz G. Lactones: Classification, Synthesis, Biological Activities, and Industrial Applications. Tetrahedron. 2021;84:132001. doi: 10.1016/j.tet.2021.132001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

