Synthetic Flavonoid BrCl-Flav-An Alternative Solution to Combat ESKAPE Pathogens
- PMID: 36290046
- PMCID: PMC9598271
- DOI: 10.3390/antibiotics11101389
Synthetic Flavonoid BrCl-Flav-An Alternative Solution to Combat ESKAPE Pathogens
Abstract
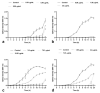
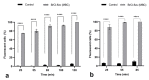
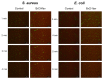
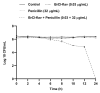
ESKAPE pathogens are considered as global threats to human health. The discovery of new molecules for which these pathogens have not yet developed resistance is a high medical priority. Synthetic flavonoids are good candidates for developing new antimicrobials. Therefore, we report here the potent in vitro antibacterial activity of BrCl-flav, a representative of a new class of synthetic tricyclic flavonoids. Minimum inhibitory/bactericidal concentration, time kill and biofilm formation assays were employed to evaluate the antibacterial potential of BrCl-flav. The mechanism of action was investigated using fluorescence and scanning electron microscopy. A checkerboard assay was used to study the effect of the tested compound in combination with antibiotics. Our results showed that BrCl-flav displayed important inhibitory activity against all tested clinical isolates, with MICs ranging between 0.24 and 125 µg/mL. A total kill effect was recorded after only 1 h of exposing Enterococcus faecium cells to BrCl-flav. Additionally, BrCl-flav displayed important biofilm disruption potential against Acinetobacter baumannii. Those effects were induced by membrane integrity damage. BrCl-flav expressed synergistic activity in combination with penicillin against a MRSA strain. Based on the potent antibacterial activity, low cytotoxicity and pro-inflammatory effect, BrCl-flav has good potential for developing new effective drugs against ESKAPE pathogens.
Keywords: ESKAPE pathogens; anti-biofilm activity; antibacterial; low cytotoxicity; pro-inflammatory effect; synergistic effect; synthetic flavonoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- WHO Regional Office for Europe. European Centre for Disease Prevention and Control . Antimicrobial Resistance Surveillance in Europe-2020 Data. WHO Regional Office for Europe; Copenhagen, Denmark: 2022.
-
- World Health Organization . Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. World Health Organization; Geneva, Switzerland: 2021.
-
- Jit M., Ng D.H.L., Luangasanatip N., Sandmann N., Atkins F., Robotham K.E., Pouwels J.V., Pouwels K.B. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: Rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020;18:38. doi: 10.1186/s12916-020-1507-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

