Glycosylation Analysis of Feline Small Intestine Following Toxoplasma gondii Infection
- PMID: 36290246
- PMCID: PMC9597833
- DOI: 10.3390/ani12202858
Glycosylation Analysis of Feline Small Intestine Following Toxoplasma gondii Infection
Abstract
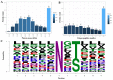
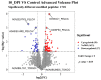
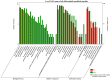
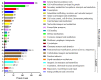
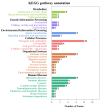
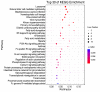
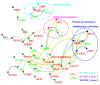
Toxoplasma gondii (T. gondii) is responsible for severe human and livestock diseases, huge economic losses, and adversely affects the health of the public and the development of animal husbandry. Glycosylation is a common posttranslational modification of proteins in eukaryotes, and N-glycosylation is closely related to the biological functions of proteins. However, glycosylation alterations in the feline small intestine following T. gondii infection have not been reported. In this study, the experimental group was intragastrically challenged with 600 brain cysts of the Prugniuad (Pru) strain that were collected from infected mice. The cats' intestinal epithelial tissues were harvested at 10 days post-infection and then sent for protein glycosylation analysis. High-performance liquid chromatography coupled to tandem mass spectrometry was used to analyze the glycosylation alterations in the small intestine of cats infected with T. gondii. The results of the present study showed that 56 glycosylated peptides were upregulated and 37 glycosylated peptides were downregulated in the feline small intestine infected by T. gondii. Additionally, we also identified eight N-glycosylated proteins of T. gondii including eight N-glycopeptides and eight N-glycosylation sites. The protein A0A086JND6_TOXGO (eEF2) and its corresponding peptide sequence were identified in T. gondii infection. Some special GO terms (i.e., cellular process and metabolic process, cell and cell part, and catalytic activity) were significantly enriched, and the Clusters of Orthologous Groups of proteins (COG) function prediction results showed that posttranslational modification, protein turnover, and chaperones (11%) had the highest enrichment for T. gondii. Interestingly, eEF2, a protein of T. gondii, is also involved in the significantly enriched T. gondii MAPK pathway. The host proteins ICAM-1 and PPT1 and the endoplasmic reticulum stress pathway may play an important role in the glycosylation of Toxoplasma-infected hosts. This is the first report showing that T. gondii oocysts can undergo N-glycosylation in the definitive host and that eEF2 is involved, which may provide a new target for T. gondii detection to prevent the spread of T. gondii oocysts in the future.
Keywords: N-glycosylation; Toxoplasma gondii; glycosylation; oocysts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Acetylome analysis of the feline small intestine following Toxoplasma gondii infection.Parasitol Res. 2020 Nov;119(11):3649-3657. doi: 10.1007/s00436-020-06880-4. Epub 2020 Sep 20. Parasitol Res. 2020. PMID: 32951143 Free PMC article.
-
Metabolomics study of cat small intestine during the early stage of Toxoplasma gondii oocyst formation identifies potential biomarkers.Vet Parasitol. 2022 Sep;309:109764. doi: 10.1016/j.vetpar.2022.109764. Epub 2022 Jul 16. Vet Parasitol. 2022. PMID: 35870221
-
Dynamic RNA profiles in the small intestinal epithelia of cats after Toxoplasma gondii infection.Infect Dis Poverty. 2023 Jul 25;12(1):68. doi: 10.1186/s40249-023-01121-z. Infect Dis Poverty. 2023. PMID: 37491273 Free PMC article.
-
Dynamics and epidemiology of Toxoplasma gondii oocyst shedding in domestic and wild felids.Transbound Emerg Dis. 2022 Sep;69(5):2412-2423. doi: 10.1111/tbed.14197. Epub 2021 Jul 15. Transbound Emerg Dis. 2022. PMID: 34153160 Review.
-
Long-Term Relationships: the Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections.Microbiol Mol Biol Rev. 2015 Dec;79(4):387-401. doi: 10.1128/MMBR.00027-15. Microbiol Mol Biol Rev. 2015. PMID: 26335719 Free PMC article. Review.
Cited by
-
Exploring Toxoplasma gondii´s Biology within the Intestinal Epithelium: intestinal-derived models to unravel sexual differentiation.Front Cell Infect Microbiol. 2023 May 29;13:1134471. doi: 10.3389/fcimb.2023.1134471. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37313339 Free PMC article. Review.
References
Grants and funding
- 32102701/National Natural Science Foundation of China
- 1610322022010/the Central Public-interest Scientific Institution Basal Research Fund
- 25-LZIHPS-05/the Innovation Project of Chinese Academy of Agricultural Sciences
- CAAS-ASTIP-2016-LVRI-03/the Agricultural Science and Technology Innovation Program (ASTIP)
- 202005AF150041/the Yunnan Expert Workstation
LinkOut - more resources
Full Text Sources
Miscellaneous

