Interleukin-10 Deficiency Impacts on TNF-Induced NFκB Regulated Responses In Vivo
- PMID: 36290283
- PMCID: PMC9598475
- DOI: 10.3390/biology11101377
Interleukin-10 Deficiency Impacts on TNF-Induced NFκB Regulated Responses In Vivo
Abstract
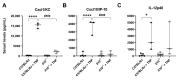
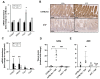
Interleukin-10 (IL-10) is an anti-inflammatory cytokine that has a major protective role against intestinal inflammation. We recently revealed that intestinal epithelial cells in vitro regulate NFκB-driven transcriptional responses to TNF via an autocrine mechanism dependent on IL-10 secretion. Here in this study, we investigated the impact of IL-10 deficiency on the NFκB pathway and its downstream targets in the small intestinal mucosa in vivo. We observed dysregulation of TNF, IκBα, and A20 gene and protein expression in the small intestine of steady-state or TNF-injected Il10-/- mice, compared to wild-type C57BL6/J counterparts. Upon TNF injection, tissue from the small intestine showed upregulation of NFκB p65[RelA] activity, which was totally diminished in Il10-/- mice and correlated with reduced levels of TNF, IκBα, and A20 expression. In serum, whilst IgA levels were noted to be markedly downregulated in IL-10-deficient- mice, normal levels of mucosal IgA were seen in intestine mucosa. Importantly, dysregulated cytokine/chemokine levels were observed in both serum and intestinal tissue lysates from naïve, as well as TNF-injected Il10-/- mice. These data further support the importance of the IL-10-canonical NFκB signaling pathway axis in regulating intestinal mucosa homeostasis and response to inflammatory triggers in vivo.
Keywords: NFκB; RelA; interleukin-10; intestine; mucosa; tumour necrosis factor.
Conflict of interest statement
All authors declare no competing commercial or financial interests in relation to the work described. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Impact of Interleukin 10 Deficiency on Intestinal Epithelium Responses to Inflammatory Signals.Front Immunol. 2021 Jun 16;12:690817. doi: 10.3389/fimmu.2021.690817. eCollection 2021. Front Immunol. 2021. PMID: 34220850 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β.PLoS One. 2012;7(6):e36890. doi: 10.1371/journal.pone.0036890. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22723832 Free PMC article.
-
Tumour necrosis factor-alpha (TNF-alpha) increases nuclear factor kappaB (NFkappaB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells.Vet Immunol Immunopathol. 2007 Mar 15;116(1-2):59-68. doi: 10.1016/j.vetimm.2006.12.008. Epub 2007 Jan 9. Vet Immunol Immunopathol. 2007. PMID: 17276517
-
Alterations of the mucosal immune system in inflammatory bowel disease.J Gastroenterol. 1996 Dec;31(6):907-16. doi: 10.1007/BF02358624. J Gastroenterol. 1996. PMID: 9027661 Review.
Cited by
-
Interleukin-10 induces TNF-driven apoptosis and ROS production in salivary gland cancer cells.Heliyon. 2024 May 29;10(11):e31777. doi: 10.1016/j.heliyon.2024.e31777. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38882335 Free PMC article.
-
Harnessing Extracellular Vesicles for Stabilized and Functional IL-10 Delivery in Macrophage Immunomodulation.bioRxiv [Preprint]. 2025 Jan 18:2025.01.14.633016. doi: 10.1101/2025.01.14.633016. bioRxiv. 2025. PMID: 39868086 Free PMC article. Preprint.
References
-
- Krause P., Morris V., Greenbaum J.A., Park Y., Bjoerheden U., Mikulski Z., Muffley T., Shui J.W., Kim G., Cheroutre H., et al. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat. Commun. 2015;6:7055. doi: 10.1038/ncomms8055. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

