Optimization of a Plasma Rich in Growth Factors Membrane for the Treatment of Inflammatory Ocular Diseases
- PMID: 36290475
- PMCID: PMC9598884
- DOI: 10.3390/bioengineering9100508
Optimization of a Plasma Rich in Growth Factors Membrane for the Treatment of Inflammatory Ocular Diseases
Abstract
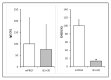
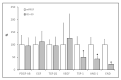
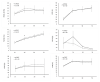
The main purpose of the present study is to develop an immunosafe fibrin membrane obtained by plasma rich in growth factors technology (is-mPRGF) with improved mechanical properties that could be applied in patients with inflammatory ocular diseases. Blood was drawn from three healthy donors and centrifuged, and the collected PRGF was activated and distributed into two groups: (i) mPRGF: a PRGF membrane maintained at 37 °C for 30 min; (ii) IS5+30: mPRGF incubated at 37 °C for 5 min and then incubated at 56 °C for 30 min. The content of both membranes was analyzed for several growth factors such as IgE and the complement activation, as well as biological activity on different ocular surface cells. Furthermore, the physical and mechanical characterizations were also evaluated. IS5+30 completely reduced the complement activity and decreased the IgE while preserving the concentration of the main growth factors. IS5+30 induced similar biological activity regarding mPRGF on the different ocular surface cells analyzed. Furthermore, no significant differences in release kinetics or fibrin degradation were observed between both membranes. Summarizing, IS5+30 totally reduces complement activity while preserving the concentration of most growth factors and their biological activity. Furthermore, the physical and mechanical properties of the fibrin membrane are preserved after heat inactivation.
Keywords: PRP; autoimmune diseases; complement system; fibrin membrane; heat inactivation; ocular surface diseases; plasma rich in growth factors; platelet rich plasma.
Conflict of interest statement
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.A. is the Scientific Director and M.d.l.F. and F.M. are scientists at BTI Biotechnology Institute, which is a company that investigates in the fields of oral implantology and PRGF-Endoret technology.
Figures

Similar articles
-
Development and optimization of a personalized fibrin membrane derived from the plasma rich in growth factors technology.Exp Eye Res. 2021 Feb;203:108402. doi: 10.1016/j.exer.2020.108402. Epub 2020 Dec 14. Exp Eye Res. 2021. PMID: 33326809
-
Effects of heat-treatment on plasma rich in growth factors-derived autologous eye drop.Exp Eye Res. 2014 Feb;119:27-34. doi: 10.1016/j.exer.2013.12.005. Epub 2013 Dec 15. Exp Eye Res. 2014. PMID: 24345372
-
Development of a new plasma rich in growth factors membrane with improved optical properties.Ann Anat. 2023 Jun;248:152071. doi: 10.1016/j.aanat.2023.152071. Epub 2023 Feb 17. Ann Anat. 2023. PMID: 36801366
-
Progress in the use of plasma rich in growth factors in ophthalmology: from ocular surface to ocular fundus.Expert Opin Biol Ther. 2022 Jan;22(1):31-45. doi: 10.1080/14712598.2021.1945030. Epub 2021 Jul 19. Expert Opin Biol Ther. 2022. PMID: 34275392 Review.
-
Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF).Curr Pharm Biotechnol. 2012 Jun;13(7):1145-52. doi: 10.2174/138920112800624382. Curr Pharm Biotechnol. 2012. PMID: 21740377 Review.
Cited by
-
Advances in Ophthalmic Engineering-Integrating Biomechanics, Tissue Engineering, and Imaging for the Future of Vision Science.Bioengineering (Basel). 2025 Apr 2;12(4):374. doi: 10.3390/bioengineering12040374. Bioengineering (Basel). 2025. PMID: 40281734 Free PMC article.
-
Assessment of corneal nerve regeneration after axotomy in a compartmentalized microfluidic chip model with automated 3D high resolution live-imaging.Front Cell Neurosci. 2024 Jul 15;18:1417653. doi: 10.3389/fncel.2024.1417653. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39076204 Free PMC article.
-
Impact of Plasma Rich in Growth Factors (PRGF) Eye Drops on Ocular Redness and Symptomatology in Patients with Dry Eye Disease.Medicina (Kaunas). 2023 May 11;59(5):928. doi: 10.3390/medicina59050928. Medicina (Kaunas). 2023. PMID: 37241160 Free PMC article.
-
Efficacy and Safety of Plasma Rich in Growth Factor in Patients with Congenital Aniridia and Dry Eye Disease.Diseases. 2024 Apr 11;12(4):76. doi: 10.3390/diseases12040076. Diseases. 2024. PMID: 38667534 Free PMC article.
-
Impact of increasingly complex cell culture conditions on the proteome of human periodontal ligament stem cells.Regen Med. 2025 Jan;20(1):21-34. doi: 10.1080/17460751.2024.2445931. Epub 2025 Jan 4. Regen Med. 2025. PMID: 39754557 Free PMC article.
References
-
- Reed G.L., Fitzgerald M.L., Polgar J. Molecular mechanisms of platelet exocytosis: Insights into the “secrete” life of thrombocytes. Blood. 2000;96:3334–3342. - PubMed
-
- Kon E., Di Matteo B., Delgado D., Cole B.J., Dorotei A., Dragoo J.L., Filardo G., Fortier L.A., Giuffrida A., Jo C.H., et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020;20:1447–1460. doi: 10.1080/14712598.2020.1798925. - DOI - PubMed
-
- Acebes-Huerta A., Arias-Fernández T., Bernardo Á., Muñoz-Turrillas M.C., Fernández-Fuertes J., Seghatchian J., Gutiérrez L. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus. Apher. Sci. 2020;59:102716. doi: 10.1016/j.transci.2019.102716. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

