Platelet-Rich Plasma Lysate-Incorporating Gelatin Hydrogel as a Scaffold for Bone Reconstruction
- PMID: 36290482
- PMCID: PMC9598158
- DOI: 10.3390/bioengineering9100513
Platelet-Rich Plasma Lysate-Incorporating Gelatin Hydrogel as a Scaffold for Bone Reconstruction
Abstract
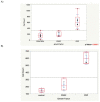
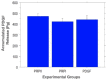
In implant dentistry, large vertical and horizontal alveolar ridge deficiencies in mandibular and maxillary bone are challenges that clinicians continue to face. One of the limitations of porous blocks for reconstruction of bone in large defects in the oral cavity, and in the musculoskeletal system, is that fibrin clot does not adequately fill the interior pores and does not persist long enough to accommodate cell migration into the center of the block. The objective of our work was to develop a gelatin-based gel incorporating platelet-rich plasma (PRP) lysate, to mimic the role that a blood clot would normally play to attract and accommodate the migration of host osteoprogenitor and endothelial cells into the scaffold, thereby facilitating bone reconstruction. A conjugate of gelatin (Gtn) and hydroxyphenyl propionic acid (HPA), an amino-acid-like molecule, was commended for this application because of its ability to undergo enzyme-mediated covalent cross-linking to form a hydrogel in vivo, after being injected as a liquid. The initiation and propagation of cross-linking were under the control of horseradish peroxidase and hydrogen peroxide, respectively. The objectives of this in vitro study were directed toward evaluating: (1) the migration of rat mesenchymal stem cells (MSCs) into Gtn-HPA gel under the influence of rat PRP lysate or recombinant platelet-derived growth factor (PDGF)-BB incorporated into the gel; (2) the differentiation of MSCs, incorporated into the gel, into osteogenic cells under the influence of PRP lysate and PDGF-BB; and (3) the release kinetics of PDGF-BB from gels incorporating two formulations of PRP lysate and recombinant PDGF-BB. Results: The number of MSCs migrating into the hydrogel was significantly (3-fold) higher in the hydrogel group incorporating PRP lysate compared to the PDGF-BB and the blank gel control groups. For the differentiation/osteogenesis assay, the osteocalcin-positive cell area percentage was significantly higher in both the gel/PRP and gel/PDGF-BB groups, compared to the two control groups: cells in the blank gels grown in cell expansion medium and in osteogenic medium. Results of the ELISA release assay indicated that Gtn-HPA acted as an effective delivery vehicle for the sustained release of PDGF-BB from two different PRP lysate batches, with about 60% of the original PDGF-BB amount in the two groups remaining in the gel at 28 days. Conclusions: Gtn-HPA accommodates MSC migration. PRP-lysate-incorporating hydrogels chemoattract increased MSC migration into the Gtn-HPA compared to the blank gel. PRP-lysate- and the PDGF-BB-incorporating gels stimulate osteogenic differentiation of the MSCs. The release of the growth factors from Gtn-HPA containing PRP lysate can extend over the period of time (weeks) necessary for bone reconstruction. The findings demonstrate that Gtn-HPA can serve as both a scaffold for cell migration and a delivery vehicle that allows sustained and controlled release of the incorporated therapeutic agent over extended periods of time. These findings commend Gtn-HPA incorporating PRP lysate for infusion into porous calcium phosphate blocks for vertical and horizontal ridge reconstruction, and for other musculoskeletal applications.
Keywords: biomaterial; bone regeneration; bone substitute; gelatin; growth factors; hydrogel; platelet-rich plasma lysate; regenerative medicine; sustainable release; tissue engineering.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Buser D., Martin W., Belser U.C. Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. Int. J. Oral Maxillofac. Implant. 2004;2:43–61. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

