Ultrafast PCR Detection of COVID-19 by Using a Microfluidic Chip-Based System
- PMID: 36290516
- PMCID: PMC9598518
- DOI: 10.3390/bioengineering9100548
Ultrafast PCR Detection of COVID-19 by Using a Microfluidic Chip-Based System
Abstract
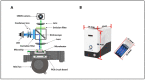
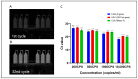
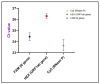
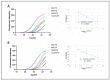
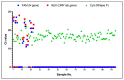
With the evolution of the pandemic caused by the Coronavirus disease of 2019 (COVID-19), reverse transcriptase-polymerase chain reactions (RT-PCR) have invariably been a golden standard in clinical diagnosis. Nevertheless, the traditional polymerase chain reaction (PCR) is not feasible for field application due to its drawbacks, such as time-consuming and laboratory-based dependence. To overcome these challenges, a microchip-based ultrafast PCR system called SWM-02 was proposed to make PCR assay in a rapid, portable, and low-cost strategy. This novel platform can perform 6-sample detection per run using multiple fluorescent channels and complete an ultrafast COVID-19 RT-PCR test within 40 min. Here, we evaluated the performance of the microdevice using the gradient-diluted COVID-19 reference samples and commercial PCR kit and determined its limit-of-detection (LoD) as 500 copies/mL, whose variation coefficients for the nucleocapsid (N) gene and open reading frame 1 ab region (ORF1ab) gene are 1.427% and 0.7872%, respectively. The system also revealed an excellent linear correlation between cycle threshold (Ct) values and dilution factors (R2 > 0.99). Additionally, we successfully detected the target RNAs and internal gene in the clinical samples by fast PCR, which shows strong consistency with conventional PCR protocol. Hence, with compact dimension, user-friendly design, and fast processing time, SWM-02 has the capability of offering timely and sensitive on-site molecular diagnosis for prevention and control of pathogen transmission.
Keywords: COVID-19; microchip-based system; microfluidics; point-of-care test; polymerase chain reaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A fully automated microfluidic PCR-array system for rapid detection of multiple respiratory tract infection pathogens.Anal Bioanal Chem. 2021 Mar;413(7):1787-1798. doi: 10.1007/s00216-021-03171-4. Epub 2021 Jan 25. Anal Bioanal Chem. 2021. PMID: 33492406 Free PMC article.
-
Performance Evaluation of a Novel Ultrafast Molecular Diagnostic Device Integrated With Microfluidic Chips and Dual Temperature Modules.Front Bioeng Biotechnol. 2022 May 19;10:895236. doi: 10.3389/fbioe.2022.895236. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35662850 Free PMC article.
-
[Evaluation of a Visually-Read Rapid Antigen Test Kit (SGA V-Chek) for Detection of SARS-CoV-2 Virus].Mikrobiyol Bul. 2021 Jul;55(3):461-464. doi: 10.5578/mb.20219815. Mikrobiyol Bul. 2021. PMID: 34416811 Turkish.
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
Rational PCR Reactor Design in Microfluidics.Micromachines (Basel). 2023 Jul 31;14(8):1533. doi: 10.3390/mi14081533. Micromachines (Basel). 2023. PMID: 37630070 Free PMC article. Review.
Cited by
-
An Aluminum-Based Microfluidic Chip for Polymerase Chain Reaction Diagnosis.Molecules. 2023 Jan 21;28(3):1085. doi: 10.3390/molecules28031085. Molecules. 2023. PMID: 36770751 Free PMC article.
-
Rapid, multiplex and automated detection of bacteria and fungi in endophthalmitis via a microfluidic real-time pcr system.J Ophthalmic Inflamm Infect. 2024 Dec 18;14(1):64. doi: 10.1186/s12348-024-00446-6. J Ophthalmic Inflamm Infect. 2024. PMID: 39694980 Free PMC article.
-
Model-Based Optimization of Solid-Supported Micro-Hotplates for Microfluidic Cryofixation.Micromachines (Basel). 2024 Aug 24;15(9):1069. doi: 10.3390/mi15091069. Micromachines (Basel). 2024. PMID: 39337729 Free PMC article.
-
Ensuring Clinical Excellence: The Mindray SAL9000 Biochemical Immunoassay System.Cell Biochem Biophys. 2025 Jun;83(2):1575-1591. doi: 10.1007/s12013-024-01568-3. Epub 2024 Oct 17. Cell Biochem Biophys. 2025. PMID: 39419930
-
Detection method for genetically modified potato using an ultra-fast PCR system.Food Sci Biotechnol. 2023 Feb 1;32(9):1-7. doi: 10.1007/s10068-023-01258-5. Online ahead of print. Food Sci Biotechnol. 2023. PMID: 36747968 Free PMC article.
References
-
- Jadhav S.A., Biji P., Panthalingal M.K., Krishna C.M., Rajkumar S., Joshi D.S., Sundaram N. Development of integrated microfluidic platform coupled with Surface-enhanced Raman Spectroscopy for diagnosis of COVID-19. Med. Hypotheses. 2021;146:110356. doi: 10.1016/j.mehy.2020.110356. - DOI - PMC - PubMed
-
- Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M., Martínez M., Poujois S., Forqu L., Valdivia A. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2021;27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

