Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice
- PMID: 36290608
- PMCID: PMC9598098
- DOI: 10.3390/antiox11101885
Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice
Abstract
Loranthus tanakae Franch. & Sav. found in China, Japan, and Korea is traditionally used for managing arthritis and respiratory diseases. In this study, we analyzed the components of L. tanakae 70% ethanol extract (LTE) and investigated the therapeutic effects of LTE on pulmonary inflammation using cells exposed to cigarette smoke condensate (CSC) and lipopolysaccharide (LPS) in vitro and in vivo in mice and performed a network analysis between components and genes based on a public database. We detected quercitrin, afzelin, rhamnetin 3-rhamnoside, and rhamnocitrin 3-rhamnoside in LTE, which induced a significant reduction in inflammatory mediators including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and inflammatory cells in CSC exposed H292 cells and in mice, accompanied by a reduction in inflammatory cell infiltration into lung tissue. In addition, LTE increased translocation into the nuclei of nuclear factor erythroid-2-related factor 2 (Nrf2). By contrast, the activation of nuclear factor (NF)-κB, induced by CSC exposure, decreased after LTE application. These results were consistent with the network pharmacological analysis. In conclusion, LTE effectively attenuated pulmonary inflammation caused by CSC+LPS exposure, which was closely involved in the enhancement of Nrf2 expression and suppression of NF-κB activation. Therefore, LTE may be a potential treatment option for pulmonary inflammatory diseases including chronic obstructive pulmonary disease (COPD).
Keywords: Loranthus tanakae Franch. & Sav.; NF-κB; Nrf2; chronic obstructive pulmonary disease; cigarette smoke condensate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




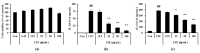




References
-
- Ko J.W., Seo C.S., Shin N.R., Kim J.S., Lee S.I., Kim J.C., Kim S.H., Shin I.S. Modified mahuang-tang, a traditional herbal medicine suppresses inflammatory responses induced by cigarette smoke in human airway epithelial cell and mice. Phytomedicine. 2019;59:152777. doi: 10.1016/j.phymed.2018.11.037. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

