S-Denitrosylation: A Crosstalk between Glutathione and Redoxin Systems
- PMID: 36290644
- PMCID: PMC9598160
- DOI: 10.3390/antiox11101921
S-Denitrosylation: A Crosstalk between Glutathione and Redoxin Systems
Abstract
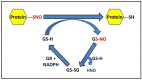
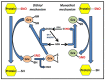
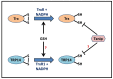
S-nitrosylation of proteins occurs as a consequence of the derivatization of cysteine thiols with nitric oxide (NO) and is often associated with diseases and protein malfunction. Aberrant S-nitrosylation, in addition to other genetic and epigenetic factors, has gained rapid importance as a prime cause of various metabolic, respiratory, and cardiac disorders, with a major emphasis on cancer and neurodegeneration. The S-nitrosoproteome, a term used to collectively refer to the diverse and dynamic repertoire of S-nitrosylated proteins, is relatively less explored in the field of redox biochemistry, in contrast to other covalently modified versions of the same set of proteins. Advancing research is gradually unveiling the enormous clinical importance of S-nitrosylation in the etiology of diseases and is opening up new avenues of prompt diagnosis that harness this phenomenon. Ever since the discovery of the two robust and highly conserved S-nitrosoglutathione reductase and thioredoxin systems as candidate denitrosylases, years of rampant speculation centered around the identification of specific substrates and other candidate denitrosylases, subcellular localization of both substrates and denitrosylases, the position of susceptible thiols, mechanisms of S-denitrosylation under basal and stimulus-dependent conditions, impact on protein conformation and function, and extrapolating these findings towards the understanding of diseases, aging and the development of novel therapeutic strategies. However, newer insights in the ever-expanding field of redox biology reveal distinct gaps in exploring the crucial crosstalk between the redoxins/major denitrosylase systems. Clarifying the importance of the functional overlap of the glutaredoxin, glutathione, and thioredoxin systems and examining their complementary functions as denitrosylases and antioxidant enzymatic defense systems are essential prerequisites for devising a rationale that could aid in predicting the extent of cell survival under high oxidative/nitrosative stress while taking into account the existence of the alternative and compensatory regulatory mechanisms. This review thus attempts to highlight major gaps in our understanding of the robust cellular redox regulation system, which is upheld by the concerted efforts of various denitrosylases and antioxidants.
Keywords: S-(de)nitrosylation; S-nitrosoglutathione; S-nitrosoproteins; antioxidant systems; glutaredoxin; glutathione; glutathione peroxidase; glutathione reductase; glutathione transferase; glutathionylation; nitric oxide; nitric oxide synthases; nitro-oxidative stress; oxidoreductases; peroxiredoxin; reactive nitrogen species; reactive oxygen species; redox homeostasis; redundancy; thiol disulfides; thioredoxin; thioredoxin interacting protein; thioredoxin reductase; thioredoxin-related protein 14.
Conflict of interest statement
The authors of this manuscript declare no conflict of interest. The authors have no financial or proprietary interest in any material discussed in this article.
Figures

References
-
- Ren X. Ph.D. Thesis. Karolinska Institutet, Department of Medical Biochemistry and Biophysics; Stockholm, Sweden: 2017. Thioredoxin and Glutaredoxin Systems under Oxidative and Nitrosative Stress.
Publication types
LinkOut - more resources
Full Text Sources

