Glutathione and Glutaredoxin in Redox Regulation and Cell Signaling of the Lens
- PMID: 36290696
- PMCID: PMC9598519
- DOI: 10.3390/antiox11101973
Glutathione and Glutaredoxin in Redox Regulation and Cell Signaling of the Lens
Abstract
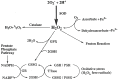
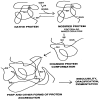
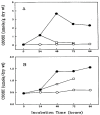
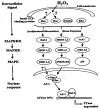
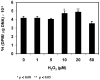
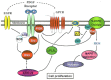
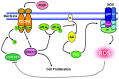
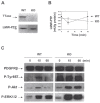
The ocular lens has a very high content of the antioxidant glutathione (GSH) and the enzymes that can recycle its oxidized form, glutathione disulfide (GSSG), for further use. It can be synthesized in the lens and, in part, transported from the neighboring anterior aqueous humor and posterior vitreous body. GSH is known to protect the thiols of the structural lens crystallin proteins from oxidation by reactive oxygen species (ROS) so the lens can maintain its transparency for proper visual function. Age-related lens opacity or senile cataract is the major visual impairment in the general population, and its cause is closely associated with aging and a constant exposure to environmental oxidative stress, such as ultraviolet light and the metabolic end product, H2O2. The mechanism for senile cataractogenesis has been hypothesized as the results of oxidation-induced protein-thiol mixed disulfide formation, such as protein-S-S-glutathione and protein-S-S-cysteine mixed disulfides, which if not reduced in time, can change the protein conformation to allow cascading modifications of various kinds leading to protein-protein aggregation and insolubilization. The consequence of such changes in lens structural proteins is lens opacity. Besides GSH, the lens has several antioxidation defense enzymes that can repair oxidation damage. One of the specific redox regulating enzymes that has been recently identified is thioltransferase (glutaredoxin 1), which works in concert with GSH, to reduce the oxidative stress as well as to regulate thiol/disulfide redox balance by preventing protein-thiol mixed disulfide accumulation in the lens. This oxidation-resistant and inducible enzyme has multiple physiological functions. In addition to protecting structural proteins and metabolic enzymes, it is able to regulate the redox signaling of the cells during growth factor-stimulated cell proliferation and other cellular functions. This review article focuses on describing the redox regulating functions of GSH and the thioltransferase enzyme in the ocular lens.
Keywords: cataract; cell proliferation; glutathione (GSH); protein-thiol mixed disulfide; reactive oxygen species (ROS); redox regulation; redox signaling; the ocular lens; thioltransferase (glutaredoxin).
Conflict of interest statement
Author declares that there is no conflict of interest.
Figures





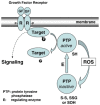
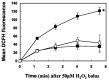
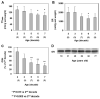
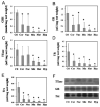
References
-
- Berman E.R. Biochemistry of the Eye. Plenum Press; New York, NY, USA: 1991. pp. 201–290.
-
- Datiles M.B., Kinoshita J.H. Pathogenesis of cataracts. In: Tasman W., Jaeger E., editors. Clinical Ophthalmology. Lippincott Press; Philadelphia, PA, USA: 1991. pp. 1–14.
-
- Augusteyn R. Protein modification in cataract: Possible oxidative mechanisms. In: Duncan G., editor. Mechanisms of Cataract Formation in the Human Lens. Academic Press; New York, NY, USA: 1981. pp. 72–115.
Publication types
LinkOut - more resources
Full Text Sources

