Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes
- PMID: 36290720
- PMCID: PMC9598584
- DOI: 10.3390/antiox11101997
Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes
Abstract
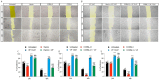
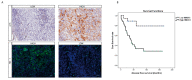
Uveal melanoma (UM) is the most common primary intraocular tumor in adults. To date, the main strategies to counteract its progression consist of focal radiation on the tumor site and ocular enucleation. Furthermore, many UM patients develop liver metastasis within 10 years following diagnosis, eventually resulting in a poorer prognosis for those patients. Dissecting the molecular mechanism involved in UM progression may lead to identify novel prognostic markers with significative clinical applications. The aim of the present study was to evaluate the role of Heme Oxygenase 1 (HO-1) in regulating UM progression. UM cell lines (92.1) were treated with Hemin (CONC e time), a strong inducer of HO-1, and VP13/47, a selective inhibitor of its enzymatic activity. Interestingly, our results showed an enhanced 92.1 cellular proliferation and wound healing ability following an HO-1 increase, overall unveiling the role played by this protein in tumor progression. Similar results were obtained following treatment with two different CO releasing molecules (CORM-3 and CORM-A1). These results were further confirmed in a clinical setting using our UM cohort. Our results demonstrated an increased median HO-1 expression in metastasizing UM when compared to nonmetastasizing patients. Overall, our results showed that HO-1 derived CO plays a major role in UM progression and HO-1 protein expression may serve as a potential prognostic and therapeutical factor in UM patients.
Keywords: cancer; carbon monoxide; heme oxygenase; proliferation; uveal melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
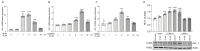


References
-
- Grisanti S., Tura A. Uveal melanoma. In: Scott J.F., Gerstenblith M.R., editors. Noncutaneous Melanoma. Codon Publications; Brisbane, QLD, Australia: 2018. - PubMed
-
- Tibullo D., Barbagallo I., Giallongo C., La Cava P., Parrinello N., Vanella L., Stagno F., Palumbo G.A., Li Volti G., Di Raimondo F. Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr. Pharm. Des. 2013;19:2765–2770. doi: 10.2174/1381612811319150012. - DOI - PubMed
-
- Spampinato M., Sferrazzo G., Pittala V., Di Rosa M., Vanella L., Salerno L., Sorrenti V., Carota G., Parrinello N., Raffaele M., et al. Non-competitive heme oxygenase-1 activity inhibitor reduces non-small cell lung cancer glutathione content and regulates cell proliferation. Mol. Biol. Rep. 2020;47:1949–1964. doi: 10.1007/s11033-020-05292-y. - DOI - PubMed
-
- Tibullo D., Barbagallo I., Giallongo C., Vanella L., Conticello C., Romano A., Saccone S., Godos J., Di Raimondo F., Li Volti G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget. 2016;7:28868–28880. doi: 10.18632/oncotarget.7563. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

