Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability
- PMID: 36290800
- PMCID: PMC9598840
- DOI: 10.3390/antiox11102077
Hemoxygenase-1 Promotes Head and Neck Cancer Cell Viability
Abstract
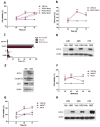
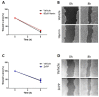
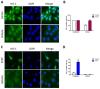
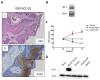
Head and neck squamous cell carcinoma (HNSCC) is a remarkably heterogeneous disease with around 50% mortality, a fact that has prompted researchers to try new approaches to improve patient survival. Hemoxygenase-1 (HO-1) is the rate-limiting step for heme degradation into carbon monoxide, free iron and biliverdin. We have previously reported that HO-1 protein is upregulated in human HNSCC samples and that it is localized in the cytoplasmic and nuclear compartments; additionally, we have demonstrated that HO-1 nuclear localization is associated with malignant progression. In this work, by using pharmacological and genetic experimental approaches, we begin to elucidate the mechanisms through which HO-1 plays a role in HNSCC. We found that high HO-1 mRNA was associated with decreased patient survival in early stages of HNSCC. In vitro experiments have shown that full-length HO-1 localizes in the cytoplasm, and that, depending on its enzymatic activity, it increases cell viability and promotes cell cycle progression. Instead, HO-1 does not alter migration capacity. Furthermore, we show that C-terminal truncated HO-1 localizes into the nucleus, increases cell viability and promotes cell cycle progression. In conclusion, we herein demonstrate that HO-1 displays protumor activities in HNSCC that depend, at least in part, on the nuclear localization of HO-1.
Keywords: cancer; head and neck; hemoxygenase-1; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

