Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition
- PMID: 36290951
- PMCID: PMC9599895
- DOI: 10.3390/bios12100814
Investigation of Spatiotemporal Profiles of Single-Pulse TMS-Evoked Potentials with Active Stimulation Compared with a Novel Sham Condition
Abstract
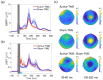
Identifying genuine cortical stimulation-elicited electroencephalography (EEG) is crucial for improving the validity and reliability of neurophysiology using transcranial magnetic stimulation (TMS) combined with EEG. In this study, we evaluated the spatiotemporal profiles of single-pulse TMS-elicited EEG response administered to the left dorsal prefrontal cortex (DLPFC) in 28 healthy participants, employing active and sham stimulation conditions. We hypothesized that the early component of TEP would be activated in active stimulation compared with sham stimulation. We specifically analyzed the (1) stimulus response, (2) frequency modulation, and (3) phase synchronization of TMS-EEG data at the sensor level and the source level. Compared with the sham condition, the active condition induced a significant increase in TMS-elicited EEG power in the 30-60 ms time interval in the stimulation area at the sensor level. Furthermore, in the source-based analysis, the active condition induced significant increases in TMS-elicited response in the 30-60 ms compared with the sham condition. Collectively, we found that the active condition could specifically activate the early component of TEP compared with the sham condition. Thus, the TMS-EEG method that was applied to the DLPFC could detect the genuine neurophysiological cortical responses by properly handling potential confounding factors such as indirect response noises.
Keywords: dorsolateral prefrontal cortex (DLPFC); electroencephalography (EEG); sham coil; source-based analysis; transcranial magnetic stimulation (TMS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Masuda F., Nakajima S., Miyazaki T., Yoshida K., Tsugawa S., Wada M., Ogyu K., Croarkin P.E., Blumberger D.M., Daskalakis Z.J., et al. Motor Cortex Excitability and Inhibitory Imbalance in Autism Spectrum Disorder Assessed with Transcranial Magnetic Stimulation: A Systematic Review. Transl. Psychiatry. 2019;9:110. doi: 10.1038/s41398-019-0444-3. - DOI - PMC - PubMed
-
- Kinjo M., Wada M., Nakajima S., Tsugawa S., Nakahara T., Blumberger D.M., Mimura M., Noda Y. Transcranial Magnetic Stimulation Neurophysiology of Patients with Major Depressive Disorder: A Systematic Review and Meta-Analysis. Psychol. Med. 2021;51:1–10. doi: 10.1017/S0033291720004729. - DOI - PMC - PubMed
-
- Mimura Y., Nishida H., Nakajima S., Tsugawa S., Morita S., Yoshida K., Tarumi R., Ogyu K., Wada M., Kurose S., et al. Neurophysiological Biomarkers Using Transcranial Magnetic Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021;121:47–59. doi: 10.1016/j.neubiorev.2020.12.003. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

