An Exosomal miRNA Biomarker for the Detection of Pancreatic Ductal Adenocarcinoma
- PMID: 36290970
- PMCID: PMC9599289
- DOI: 10.3390/bios12100831
An Exosomal miRNA Biomarker for the Detection of Pancreatic Ductal Adenocarcinoma
Abstract
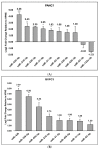
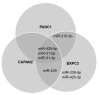
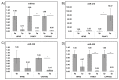
Pancreatic ductal adenocarcinoma (PDAC) remains a difficult tumor to diagnose and treat. To date, PDAC lacks routine screening with no markers available for early detection. Exosomes are 40-150 nm-sized extracellular vesicles that contain DNA, RNA, and proteins. These exosomes are released by all cell types into circulation and thus can be harvested from patient body fluids, thereby facilitating a non-invasive method for PDAC detection. A bioinformatics analysis was conducted utilizing publicly available miRNA pancreatic cancer expression and genome databases. Through this analysis, we identified 18 miRNA with strong potential for PDAC detection. From this analysis, 10 (MIR31, MIR93, MIR133A1, MIR210, MIR330, MIR339, MIR425, MIR429, MIR1208, and MIR3620) were chosen due to high copy number variation as well as their potential to differentiate patients with chronic pancreatitis, neoplasms, and PDAC. These 10 were examined for their mature miRNA expression patterns, giving rise to 18 mature miRs for further analysis. Exosomal RNA from cell culture media was analyzed via RTqPCR and seven mature miRs exhibited statistical significance (miR-31-5p, miR-31-3p, miR-210-3p, miR-339-5p, miR-425-5p, miR-425-3p, and miR-429). These identified biomarkers can potentially be used for early detection of PDAC.
Keywords: biomarker; cancer; diagnostics; exosomes; miRNA; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- NIH R15AI127214/National Institute of Health
- Institute for Sensing and Embedded Networking Systems Engineering (I-SENSE) Research Initiative Award/Florida Atlantic University
- FAU Faculty Mentoring Award/Florida Atlantic University
- Start-up research support from College of Engineering and Computer Science/Florida Atlantic University
LinkOut - more resources
Full Text Sources
Medical

