All-Polymeric Electrode Based on PEDOT:PSS for In Vivo Neural Recording
- PMID: 36290990
- PMCID: PMC9599788
- DOI: 10.3390/bios12100853
All-Polymeric Electrode Based on PEDOT:PSS for In Vivo Neural Recording
Abstract
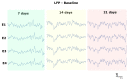
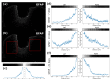
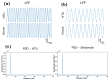
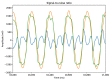
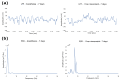
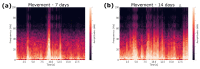
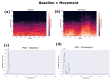
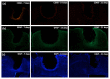
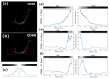
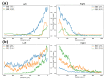
One of the significant challenges today in the brain-machine interfaces that use invasive methods is the stability of the chronic record. In recent years, polymer-based electrodes have gained notoriety for achieving mechanical strength values close to that of brain tissue, promoting a lower immune response to the implant. In this work, we fabricated fully polymeric electrodes based on PEDOT:PSS for neural recording in Wistar rats. We characterized the electrical properties and both in vitro and in vivo functionality of the electrodes. Additionally, we employed histological processing and microscopical visualization to evaluate the tecidual immune response at 7, 14, and 21 days post-implant. Electrodes with 400-micrometer channels showed a 12 dB signal-to-noise ratio. Local field potentials were characterized under two conditions: anesthetized and free-moving. There was a proliferation of microglia at the tissue-electrode interface in the early days, though there was a decrease after 14 days. Astrocytes also migrated to the interface, but there was not continuous recruitment of these cells in the tissue; there was inflammatory stability by day 21. The signal was not affected by this inflammatory action, demonstrating that fully polymeric electrodes can be an alternative means to prolong the valuable time of neural recordings.
Keywords: BMI; PEDOT:PSS; immune response; neural recording.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

