SARS-CoV-2-on-Chip for Long COVID Management
- PMID: 36291027
- PMCID: PMC9599615
- DOI: 10.3390/bios12100890
SARS-CoV-2-on-Chip for Long COVID Management
Abstract
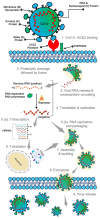
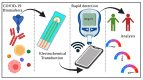
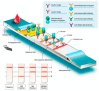
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a "wicked evil" in this century due to its extended progression and huge human mortalities. Although the diagnosis of SARS-CoV-2 viral infection is made simple and practical by employing reverse transcription polymerase chain reaction (RT-PCR) investigation, the process is costly, complex, time-consuming, and requires experts for testing and the constraints of a laboratory. Therefore, these challenges have raised the paradigm of on-site portable biosensors on a single chip, which reduces human resources and enables remote access to minimize the overwhelming burden on the existing global healthcare sector. This article reviews the recent advancements in biosensors for long coronavirus disease (COVID) management using a multitude of devices, such as point-of-care biosensors and lab-on-chip biosensors. Furthermore, it details the shift in the paradigm of SARS-CoV-2-on-chip biosensors from the laboratory to on-site detection with intelligent and economical operation, representing near-future diagnostic technologies for public health emergency management.
Keywords: RT-PCR; SARS-CoV-2-on-chip; biosensors; long COVID; point-of-care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





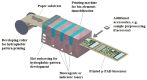

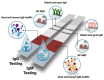
References
-
- Siavash I. Nano and biosensors for the detection of SARS-CoV-2: Challenges and opportunities. Mater. Adv. 2020;1:3092.
-
- Chaudhary V., Kaushik A., Furukawa H., Khosla A. Review—Towards 5th generation AI and IoT driven sustainable intelligent sensors based on 2D mxenes and borophene. ECS Sens. Plus. 2022;1:013601. doi: 10.1149/2754-2726/ac5ac6. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

