Biomarker-Targeted Therapies in Non-Small Cell Lung Cancer: Current Status and Perspectives
- PMID: 36291069
- PMCID: PMC9600447
- DOI: 10.3390/cells11203200
Biomarker-Targeted Therapies in Non-Small Cell Lung Cancer: Current Status and Perspectives
Abstract
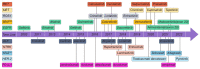
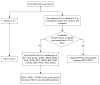
Non-small-cell lung cancer (NSCLC) is one of the most common malignancies and the leading causes of cancer-related death worldwide. Despite many therapeutic advances in the past decade, NSCLC remains an incurable disease for the majority of patients. Molecular targeted therapies and immunotherapies have significantly improved the prognosis of NSCLC. However, the vast majority of advanced NSCLC develop resistance to current therapies and eventually progress. In this review, we discuss current and potential therapies for NSCLC, focusing on targeted therapies and immunotherapies. We highlight the future role of metabolic therapies and combination therapies in NSCLC.
Keywords: immunotherapies; non-small-cell lung cancer; targeted therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

