Modulation of Neutrophil Activity by Soluble Complement Cleavage Products-An In-Depth Analysis
- PMID: 36291163
- PMCID: PMC9600402
- DOI: 10.3390/cells11203297
Modulation of Neutrophil Activity by Soluble Complement Cleavage Products-An In-Depth Analysis
Abstract
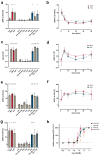
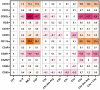
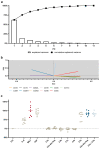
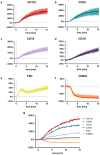
The cellular and fluid phase-innate immune responses of many diseases predominantly involve activated neutrophil granulocytes and complement factors. However, a comparative systematic analysis of the early impact of key soluble complement cleavage products, including anaphylatoxins, on neutrophil granulocyte function is lacking. Neutrophil activity was monitored by flow cytometry regarding cellular (electro-)physiology, cellular activity, and changes in the surface expression of activation markers. The study revealed no major effects induced by C3a or C4a on neutrophil functions. By contrast, exposure to C5a or C5a des-Arg stimulated neutrophil activity as reflected in changes in membrane potential, intracellular pH, glucose uptake, and cellular size. Similarly, C5a and C5a des-Arg but no other monitored complement cleavage product enhanced phagocytosis and reactive oxygen species generation. C5a and C5a des-Arg also altered the neutrophil surface expression of several complement receptors and neutrophil activation markers, including C5aR1, CD62L, CD10, and CD11b, among others. In addition, a detailed characterization of the C5a-induced effects was performed with a time resolution of seconds. The multiparametric response of neutrophils was further analyzed by a principal component analysis, revealing CD11b, CD10, and CD16 to be key surrogates of the C5a-induced effects. Overall, we provide a comprehensive insight into the very early interactions of neutrophil granulocytes with activated complement split products and the resulting neutrophil activity. The results provide a basis for a better and, importantly, time-resolved and multiparametric understanding of neutrophil-related (patho-)physiologies.
Keywords: anaphylatoxins; complement system; inflammation; innate immunity; neutrophils.
Conflict of interest statement
C.Q.S. is an inventor of patent applications that describes the use of engineered complement inhibitors for therapeutic applications. He has received honoraria for speaking at symposia as well as research funding from the pharmaceutical industry Otherwise, the authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

