A Microfluidic In Vitro Three-Dimensional Dynamic Model of the Blood-Brain Barrier to Study the Transmigration of Immune Cells
- PMID: 36291227
- PMCID: PMC9599663
- DOI: 10.3390/brainsci12101293
A Microfluidic In Vitro Three-Dimensional Dynamic Model of the Blood-Brain Barrier to Study the Transmigration of Immune Cells
Abstract
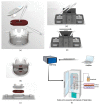
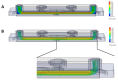
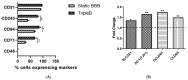
To study the biodistribution of new chemical and biological entities, an in vitro model of the blood-brain barrier (BBB) may become an essential tool during early phases of drug discovery. Here, we present a proof-of-concept of an in-house designed three-dimensional BBB biochip designed by us. This three-dimensional dynamic BBB model consists of endothelial cells and astrocytes, co-cultured on opposing sides of a polymer-coated membrane under flow mimicking blood flow. Our results demonstrate a highly effective BBB as evidenced by (i) a 30-fold increase in transendothelial electrical resistance (TEER), (ii) a significantly higher expression of tight junction proteins, and (iii) the low FITC-dextran permeability of our technical solution as compared to a static in vitro BBB model. Importantly, our three-dimensional BBB model effectively expresses P-glycoprotein (Pg-p), a hallmark characteristic for brain-derived endothelial cells. In conclusion, we provide here a complete holistic approach and insight to the whole BBB system, potentially delivering translational significance in the clinical and pharmaceutical arenas.
Keywords: FITC-dextran permeability; P-glycoprotein; TripleB slides; blood–brain barrier; central nervous system; endothelial cells; microfluidic device; transendothelial electrical resistance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening.Biotechnol Bioeng. 2017 Jan;114(1):184-194. doi: 10.1002/bit.26045. Epub 2016 Jul 21. Biotechnol Bioeng. 2017. PMID: 27399645 Free PMC article.
-
Hybrid elastomer-plastic microfluidic device as a convenient model for mimicking the blood-brain barrier in vitro.Biomed Microdevices. 2019 Nov 4;21(4):90. doi: 10.1007/s10544-019-0446-1. Biomed Microdevices. 2019. PMID: 31686217
-
Real-time acquisition of transendothelial electrical resistance in an all-human, in vitro, 3-dimensional, blood-brain barrier model exemplifies tight-junction integrity.FASEB J. 2018 Jan;32(1):168-182. doi: 10.1096/fj.201700162R. Epub 2017 Sep 7. FASEB J. 2018. PMID: 28883042 Free PMC article.
-
A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes.Neurochem Int. 2009 Mar-Apr;54(3-4):253-63. doi: 10.1016/j.neuint.2008.12.002. Epub 2008 Dec 7. Neurochem Int. 2009. PMID: 19111869
-
Review Article: Capturing the physiological complexity of the brain's neuro-vascular unit in vitro.Biomicrofluidics. 2018 Oct 16;12(5):051502. doi: 10.1063/1.5045126. eCollection 2018 Sep. Biomicrofluidics. 2018. PMID: 30364144 Free PMC article. Review.
Cited by
-
Towards Novel Biomimetic In Vitro Models of the Blood-Brain Barrier for Drug Permeability Evaluation.Bioengineering (Basel). 2023 May 10;10(5):572. doi: 10.3390/bioengineering10050572. Bioengineering (Basel). 2023. PMID: 37237642 Free PMC article. Review.
-
Multiple sclerosis: etiology in the context of neurovascular unit and immune system involvement and advancements with in vitro blood-brain barrier models.Front Immunol. 2025 Jun 10;16:1595276. doi: 10.3389/fimmu.2025.1595276. eCollection 2025. Front Immunol. 2025. PMID: 40557144 Free PMC article. Review.
-
Modular cone-and-plate device for mechanofluidic assays in Transwell inserts.Front Bioeng Biotechnol. 2025 Jan 27;13:1494553. doi: 10.3389/fbioe.2025.1494553. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 39931136 Free PMC article.
-
Hybrid-integrated devices for mimicking malignant brain tumors ("tumor-on-a-chip") for in vitro development of targeted drug delivery and personalized therapy approaches.Front Med (Lausanne). 2024 Nov 19;11:1452298. doi: 10.3389/fmed.2024.1452298. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39629230 Free PMC article. Review.
-
A Cost-Effective and Easy to Assemble 3D Human Microchannel Blood-Brain Barrier Model and Its Application in Tumor Cell Adhesion Under Flow.Cells. 2025 Mar 19;14(6):456. doi: 10.3390/cells14060456. Cells. 2025. PMID: 40136705 Free PMC article.
References
-
- Deuschl G., Beghi E., Fazekas F., Varga T., Christoforidi K.A., Sipido E., Bassetti C.L., Vos T., Feigin V.L. The Burden of Neurological Diseases in Europe: An Analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–e567. doi: 10.1016/S2468-2667(20)30190-0. - DOI - PubMed
-
- Feigin V.L., Abajobir A.A., Abate K.H., Abd-Allah F., Abdulle A.M., Abera S.F., Abyu G.Y., Ahmed M.B., Aichour A.N., Aichour I. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

