Human-in-the-Loop Optimization of Transcranial Electrical Stimulation at the Point of Care: A Computational Perspective
- PMID: 36291228
- PMCID: PMC9599464
- DOI: 10.3390/brainsci12101294
Human-in-the-Loop Optimization of Transcranial Electrical Stimulation at the Point of Care: A Computational Perspective
Abstract
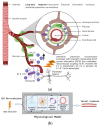
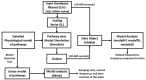
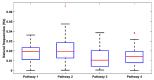
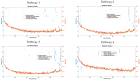
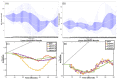
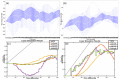
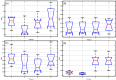
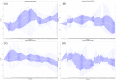
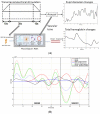
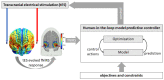
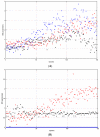
Individual differences in the responsiveness of the brain to transcranial electrical stimulation (tES) are increasingly demonstrated by the large variability in the effects of tES. Anatomically detailed computational brain models have been developed to address this variability; however, static brain models are not “realistic” in accounting for the dynamic state of the brain. Therefore, human-in-the-loop optimization at the point of care is proposed in this perspective article based on systems analysis of the neurovascular effects of tES. First, modal analysis was conducted using a physiologically detailed neurovascular model that found stable modes in the 0 Hz to 0.05 Hz range for the pathway for vessel response through the smooth muscle cells, measured with functional near-infrared spectroscopy (fNIRS). During tES, the transient sensations can have arousal effects on the hemodynamics, so we present a healthy case series for black-box modeling of fNIRS−pupillometry of short-duration tDCS effects. The block exogeneity test rejected the claim that tDCS is not a one-step Granger cause of the fNIRS total hemoglobin changes (HbT) and pupil dilation changes (p < 0.05). Moreover, grey-box modeling using fNIRS of the tDCS effects in chronic stroke showed the HbT response to be significantly different (paired-samples t-test, p < 0.05) between the ipsilesional and contralesional hemispheres for primary motor cortex tDCS and cerebellar tDCS, which was subserved by the smooth muscle cells. Here, our opinion is that various physiological pathways subserving the effects of tES can lead to state−trait variability, which can be challenging for clinical translation. Therefore, we conducted a case study on human-in-the-loop optimization using our reduced-dimensions model and a stochastic, derivative-free covariance matrix adaptation evolution strategy. We conclude from our computational analysis that human-in-the-loop optimization of the effects of tES at the point of care merits investigation in future studies for reducing inter-subject and intra-subject variability in neuromodulation.
Keywords: functional near-infrared spectroscopy; model predictive control; pupillometry; systems analysis; transcranial electrical stimulation.
Conflict of interest statement
Yashika Arora declares that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. Anirban Dutta is an inventor of the intellectual property developed by SIAGNOS LLC, USA, where Anirban Dutta holds equity.
Figures


References
-
- Chan L., Liao Y.-C., Mo G.B., Dudley J.J., Cheng C.-L., Kristensson P.O., Oulasvirta A. Investigating Positive and Negative Qualities of Human-in-the-Loop Optimization for Designing Interaction Techniques; Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems; New Orleans, LA, USA. 30 April–5 May 2022; New York, NY, USA: Association for Computing Machinery; 2022. pp. 1–14.
LinkOut - more resources
Full Text Sources

