Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI
- PMID: 36291295
- PMCID: PMC9599333
- DOI: 10.3390/brainsci12101361
Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI
Abstract
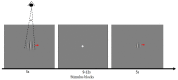
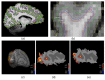
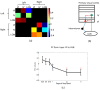
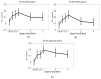
Layer-specific cortical microcircuits have been explored through invasive animal studies, yet it is not possible to reliably characterize them functionally and noninvasively in the human brain. However, recent advances in ultra-high-field functional magnetic resonance imaging (fMRI) have made it feasible to reasonably resolve layer-specific fMRI signals with sub-millimeter resolution. Here, we propose an experimental and analytical framework that enables the noninvasive functional characterization of layer-specific cortical microcircuits. Specifically, we illustrate this framework by characterizing layer-specific functional pathways in the corticogeniculate network of the human visual system by obtaining sub-millimeter fMRI at 7T using a task which engages the magnocellular pathway between the lateral geniculate nucleus (LGN) and the primary visual cortex. Our results demonstrate that: (i) center-surround inhibition in magnocellular neurons within LGN is detectable using localized fMRI responses; (ii) feedforward (LGN → layers VI/IV, layer IV → layer VI) and feedback (layer VI → LGN) functional pathways, known to exist from invasive animal studies, can be inferred using dynamic directional connectivity models of fMRI and could potentially explain the mechanism underlying center-surround inhibition as well as gain control by layer VI in the human visual system. Our framework is domain-neutral and could potentially be employed to investigate the layer-specific cortical microcircuits in other systems related to cognition, memory and language.
Keywords: center-surround inhibition; cortical layers; corticogeniculate feedback; dynamic directional connectivity; high-resolution 7T fMRI; layer-specific fMRI; magnocellular lateral geniculate neurons; primary visual cortex.
Conflict of interest statement
The authors declare that they have no conflict of interest with respect to this work.
Figures




Similar articles
-
Robust functional mapping of layer-selective responses in human lateral geniculate nucleus with high-resolution 7T fMRI.Proc Biol Sci. 2020 Apr 29;287(1925):20200245. doi: 10.1098/rspb.2020.0245. Epub 2020 Apr 15. Proc Biol Sci. 2020. PMID: 32290803 Free PMC article.
-
Distinct patterns of corticogeniculate feedback to different layers of the lateral geniculate nucleus.Eye Brain. 2014 Sep;2014(6 Suppl 1):57-73. doi: 10.2147/EB.S64281. Eye Brain. 2014. PMID: 25892906 Free PMC article.
-
Resting state fMRI connectivity is sensitive to laminar connectional architecture in the human brain.Brain Inform. 2022 Jan 17;9(1):2. doi: 10.1186/s40708-021-00150-4. Brain Inform. 2022. PMID: 35038072 Free PMC article.
-
Mapping the primate lateral geniculate nucleus: a review of experiments and methods.J Physiol Paris. 2014 Feb;108(1):3-10. doi: 10.1016/j.jphysparis.2013.10.001. Epub 2013 Nov 21. J Physiol Paris. 2014. PMID: 24270042 Free PMC article. Review.
-
Laminar functional magnetic resonance imaging in vision research.Front Neurosci. 2022 Oct 4;16:910443. doi: 10.3389/fnins.2022.910443. eCollection 2022. Front Neurosci. 2022. PMID: 36267240 Free PMC article. Review.
Cited by
-
Visual motion thresholds mapped to midget and parasol ganglion cell topography in the human retina.Sci Rep. 2025 Sep 1;15(1):32254. doi: 10.1038/s41598-025-16986-3. Sci Rep. 2025. PMID: 40890279 Free PMC article.
References
LinkOut - more resources
Full Text Sources

