Odor Pleasantness Modulates Functional Connectivity in the Olfactory Hedonic Processing Network
- PMID: 36291341
- PMCID: PMC9599424
- DOI: 10.3390/brainsci12101408
Odor Pleasantness Modulates Functional Connectivity in the Olfactory Hedonic Processing Network
Abstract
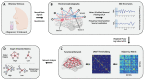
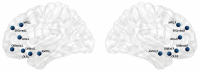
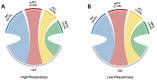
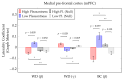
Olfactory hedonic evaluation is the primary dimension of olfactory perception and thus central to our sense of smell. It involves complex interactions between brain regions associated with sensory, affective and reward processing. Despite a recent increase in interest, several aspects of olfactory hedonic evaluation remain ambiguous: uncertainty surrounds the communication between, and interaction among, brain areas during hedonic evaluation of olfactory stimuli with different levels of pleasantness, as well as the corresponding supporting oscillatory mechanisms. In our study we investigated changes in functional interactions among brain areas in response to odor stimuli using electroencephalography (EEG). To this goal, functional connectivity networks were estimated based on phase synchronization between EEG signals using the weighted phase lag index (wPLI). Graph theoretic metrics were subsequently used to quantify the resulting changes in functional connectivity of relevant brain regions involved in olfactory hedonic evaluation. Our results indicate that odor stimuli of different hedonic values evoke significantly different interaction patterns among brain regions within the olfactory cortex, as well as in the anterior cingulate and orbitofrontal cortices. Furthermore, significant hemispheric laterality effects have been observed in the prefrontal and anterior cingulate cortices, specifically in the beta ((13-30) Hz) and gamma ((30-40) Hz) frequency bands.
Keywords: brain connectivity; electroencephalography; hedonic evaluation; lateralization; olfaction.
Conflict of interest statement
Junji Hamano and Mariana Saba are research investigators with Procter & Gamble International Operations, Singapore. This study has been funded through a grant from A*STAR (Singapore) and Procter & Gamble.
Figures



Similar articles
-
Community Analysis of Brain Functional Networks Reveals Systems-Level Integration in Olfactory Hedonic Perception.Annu Int Conf IEEE Eng Med Biol Soc. 2021 Nov;2021:5995-5998. doi: 10.1109/EMBC46164.2021.9629919. Annu Int Conf IEEE Eng Med Biol Soc. 2021. PMID: 34892484
-
Cortical network and connectivity underlying hedonic olfactory perception.J Neural Eng. 2021 Oct 11;18(5). doi: 10.1088/1741-2552/ac28d2. J Neural Eng. 2021. PMID: 34547740
-
Development of Odor Hedonics: Experience-Dependent Ontogeny of Circuits Supporting Maternal and Predator Odor Responses in Rats.J Neurosci. 2016 Jun 22;36(25):6634-50. doi: 10.1523/JNEUROSCI.0632-16.2016. J Neurosci. 2016. PMID: 27335397 Free PMC article.
-
Cortical odor processing in health and disease.Prog Brain Res. 2014;208:275-305. doi: 10.1016/B978-0-444-63350-7.00011-5. Prog Brain Res. 2014. PMID: 24767487 Free PMC article. Review.
-
Functional MRI of congenital hyposmia: brain activation to odors and imagination of odors and tastes.J Comput Assist Tomogr. 2002 Jan-Feb;26(1):39-61. doi: 10.1097/00004728-200201000-00008. J Comput Assist Tomogr. 2002. PMID: 11801904 Review.
Cited by
-
Unpleasant odors compared to pleasant ones cause higher cortical activations detectable by fNIRS and observable mostly in females.APL Bioeng. 2025 Jan 7;9(1):016101. doi: 10.1063/5.0231217. eCollection 2025 Mar. APL Bioeng. 2025. PMID: 39801499 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

