From Prescription Drugs to Natural Health Products: Medication Use in Canadian Infants
- PMID: 36291411
- PMCID: PMC9600175
- DOI: 10.3390/children9101475
From Prescription Drugs to Natural Health Products: Medication Use in Canadian Infants
Abstract
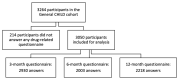
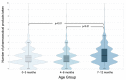
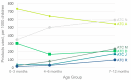
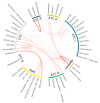
Limited data exist on pharmaceutical product use by infants, although available data suggests higher prevalence of use among children under 12 months of age. We conducted a descriptive study of 3050 infants recruited in the CHILD Cohort Study, a prospective, multicenter, longitudinal cohort following children from pregnancy through childhood. Parents were surveyed for use of prescription and over-the-counter drugs, and natural health products (NHPs, including homeopathic products and vitamins) at 3, 6, and 12 months after delivery. By one year of age, 96.0% of children had taken at least one pharmaceutical product. Among 307 reported products, 32 were given to at least 1% of cohort infants. Vitamin D, acetaminophen, ibuprofen, topical hydrocortisone, amoxicillin, and nystatin were the most common medications and natural health products (NHPs) received, with 8/32 of the most frequently used products being NHPs. Overall, 14.7% of pharmaceutical products administered to children were off-label and 35.8% were NHPs or products without a Drug Identification Number (DIN). The use of over-the-counter medications and NHPs is common and off-label use of drugs is frequent, even in the first year of life. This study highlights the importance of conducting studies on medication use in infants, and of infant medication use monitoring by healthcare providers.
Keywords: drugs; family medicine; general practice; medicine and pharmaceutical industry; pediatrics; pharmacy; primary care.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study, the collection, analyses and interpretation of data, writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Pharmaceutical counseling: Between evidence-based medicine and profits.Int J Risk Saf Med. 2015;27 Suppl 1:S87-8. doi: 10.3233/JRS-150701. Int J Risk Saf Med. 2015. PMID: 26639727
-
Use of natural health products in children: survey of parents in waiting rooms.Can Fam Physician. 2013 Aug;59(8):e364-71. Can Fam Physician. 2013. PMID: 23946043 Free PMC article.
-
The use of natural health products by paediatric patients in respite care.Paediatr Child Health. 2015 Jan-Feb;20(1):23-9. doi: 10.1093/pch/20.1.23. Paediatr Child Health. 2015. PMID: 25722640 Free PMC article.
-
Interventions for infantile seborrhoeic dermatitis (including cradle cap).Cochrane Database Syst Rev. 2019 Mar 4;3(3):CD011380. doi: 10.1002/14651858.CD011380.pub2. Cochrane Database Syst Rev. 2019. PMID: 30828791 Free PMC article.
-
Unlicensed and off-label prescription of drugs to children in primary health care: A systematic review.J Evid Based Med. 2020 Nov;13(4):292-300. doi: 10.1111/jebm.12402. Epub 2020 Oct 13. J Evid Based Med. 2020. PMID: 33047516
Cited by
-
Infant Vitamin D Supplements, Fecal Microbiota and Their Metabolites at 3 Months of Age in the CHILD Study Cohort.Biomolecules. 2023 Jan 19;13(2):200. doi: 10.3390/biom13020200. Biomolecules. 2023. PMID: 36830570 Free PMC article.
References
-
- Taine M., Offredo L., Dray-Spira R., Weill A., Chalumeau M., Zureik M. Paediatric outpatient prescriptions in France between 2010 and 2019: A nationwide population-based study: Paediatric outpatient prescriptions in France, 2010 to 2019. Lancet Reg. Health Eur. 2021;7:100129. doi: 10.1016/j.lanepe.2021.100129. - DOI - PMC - PubMed
-
- Carnovale C., Conti V., Perrone V., Antoniazzi S., Pozzi M., Merlino M., Venegoni M., Clementi E., Radice S. Paediatric drug use with focus on off-label prescriptions in Lombardy and implications for therapeutic approaches. Eur. J. Pediatr. 2013;172:1679–1685. doi: 10.1007/s00431-013-2111-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

