Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study
- PMID: 36291452
- PMCID: PMC9600699
- DOI: 10.3390/children9101516
Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study
Abstract
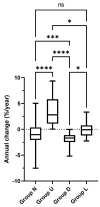
Background: Childhood asthma is a major risk for low lung function in later adulthood, but what factors in asthma are associated with the poor lung function during childhood is not known. Objective: To identify clinical factors in children with asthma associated with low or declining lung function during the treatment. Methods: We enrolled children with asthma who had been treated throughout three age periods, i.e., 6−9, 10−12, and 13−15 years old, at seven specialized hospitals in Japan. Clinical information and lung function measurements were retrieved from the electronic chart systems. To characterize the lung function trajectories during each age period, we evaluated the forced expiratory volume 1 (FEV1) with % predicted values and individual changes by the slope (S) from linear regression. We defined four trajectory patterns: normal (Group N) and low (Group L), showing %FEV1 ≥80% or <80% throughout all three periods; upward (Group U) and downward (Group D), showing S ≥ 0 or S < 0%. Logistic regression analysis was performed to compare factors associated with the unfavorable (D/L) versus favorable (N/U) groups. Results: Among 273 eligible patients, 197 (72%) were classified into Group N (n = 150)/U (n = 47), while 76 (28%) were in Group D (n = 66)/L (n = 10). A history of poor asthma control, long-acting beta2 agonist use, and a lower height Z-score during 13−15 years were associated with an unfavorable outcome (Group D/L). Conversely, inhaled corticosteroid (ICS) use during 10−12 years and high-dose ICS use during 13−15 years were associated with a favorable outcome (Group N/U). Conclusion: We identified several factors that are associated with unfavorable lung function changes in pediatric asthma. Attention should be paid to the possible relationship between yearly changes in lung function and poor asthma control, use of ICS (and its dose) and use of LABA.
Keywords: asthma; asthma control; childhood and adolescence; inhaled corticosteroids; lung function; risk factors.
Conflict of interest statement
T.F. is a consultant for B.M.L. and has received lecture fees from Maruho, Torii, Sanofi, G.S.K. and Torii Pharmaceutical. S.K. has received a lecture fee from Kyorin Pharmaceutical, Mylan Seiyaku, and Torii Pharmaceutical. The other authors declare they have no conflict of interest.
Figures

Similar articles
-
Lung function decline before and after treatment of World Trade Center associated obstructive airways disease with inhaled corticosteroids and long-acting beta agonists.Am J Ind Med. 2021 Oct;64(10):853-860. doi: 10.1002/ajim.23272. Epub 2021 Jul 13. Am J Ind Med. 2021. PMID: 34254700 Free PMC article.
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Evid Based Child Health. 2014 Dec;9(4):931-1046. doi: 10.1002/ebch.1989. Evid Based Child Health. 2014. PMID: 25504973
-
Bronchial reversibility with a short-acting β2-agonist predicts the FEV1 response to administration of a long-acting β2-agonist with inhaled corticosteroids in patients with bronchial asthma.Exp Ther Med. 2011 Jul;2(4):619-623. doi: 10.3892/etm.2011.268. Epub 2011 May 12. Exp Ther Med. 2011. PMID: 22977550 Free PMC article.
-
Extrafine HFA-beclomethasone-formoterol vs. nonextrafine combination of an inhaled corticosteroid and a long acting β2-agonist in patients with persistent asthma: A systematic review and meta-analysis.PLoS One. 2021 Sep 3;16(9):e0257075. doi: 10.1371/journal.pone.0257075. eCollection 2021. PLoS One. 2021. PMID: 34478483 Free PMC article.
-
Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2019 Mar 6;3(3):CD012930. doi: 10.1002/14651858.CD012930.pub2. Cochrane Database Syst Rev. 2019. PMID: 30839102 Free PMC article.
Cited by
-
Efficacy evaluation of allergen-specific immunotherapy in children with asthma: a systematic review and meta-analysis.BMC Pulm Med. 2025 Jul 2;25(1):293. doi: 10.1186/s12890-025-03763-1. BMC Pulm Med. 2025. PMID: 40604821 Free PMC article.
References
-
- Ross J.C., Castaldi P.J., Cho M.H., Hersh C.P., Rahaghi F.N., Sanchez-Ferrero G.V., Parker M.M., Litonjua A.A., Sparrow D., Dy J.G., et al. Longitudinal modeling of lung function trajectories in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018;198:1033–1042. doi: 10.1164/rccm.201707-1405OC. - DOI - PMC - PubMed
-
- Bui D.S., Lodge C.J., Perret J.L., Lowe A., Hamilton G.S., Thompson B., Giles G., Tan D., Erbas B., Pirkis J., et al. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: A prospective cohort study. Lancet Respir. Med. 2021;9:387–396. doi: 10.1016/S2213-2600(20)30413-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

