PHLPP Inhibitor NSC74429 Is Neuroprotective in Rodent Models of Cardiac Arrest and Traumatic Brain Injury
- PMID: 36291561
- PMCID: PMC9599532
- DOI: 10.3390/biom12101352
PHLPP Inhibitor NSC74429 Is Neuroprotective in Rodent Models of Cardiac Arrest and Traumatic Brain Injury
Abstract
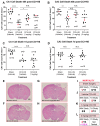
Pleckstrin homology domain and leucine rich repeat protein phosphatase (PHLPP) knockout mice have improved outcomes after a stroke, traumatic brain injury (TBI), and decreased maladaptive vascular remodeling following vascular injury. Thus, small-molecule PHLPP inhibitors have the potential to improve neurological outcomes in a variety of conditions. There is a paucity of data on the efficacy of the known experimental PHLPP inhibitors, and not all may be suited for targeting acute brain injury. Here, we assessed several PHLPP inhibitors not previously explored for neuroprotection (NSC13378, NSC25247, and NSC74429) that had favorable predicted chemistries for targeting the central nervous system (CNS). Neuronal culture studies in staurosporine (apoptosis), glutamate (excitotoxicity), and hydrogen peroxide (necrosis/oxidative stress) revealed that NSC74429 at micromolar concentrations was the most neuroprotective. Subsequent testing in a rat model of asphyxial cardiac arrest, and in a mouse model of severe TBI, showed that serial dosing of 1 mg/kg of NSC74429 over 3 days improved hippocampal survival in both models. Taken together, NSC74429 is neuroprotective across multiple insult mechanisms. Future pharmacokinetic and pharmacodynamic (PK/PD) studies are warranted to optimize dosing, and mechanistic studies are needed to determine the percentage of neuroprotection mediated by PHLPP1/2 inhibition, or potentially from the modulation of PHLPP-independent targets.
Keywords: NSC74429; PHLPP; PHLPP1; PHLPP2; brain; neuroprotection.
Conflict of interest statement
Travis C. Jackson and Patrick M. Kochanek are co-inventors on a pending patent application titled: “Compounds for the treatment of acute organ injury” (USPTO Application No. 16/584,314).
Figures



References
-
- Huang J., Cai C., Zheng T., Wu X., Wang D., Zhang K., Xu B., Yan R., Gong H., Zhang J., et al. Endothelial Scaffolding Protein ENH (Enigma Homolog Protein) Promotes PHLPP2 (Pleckstrin Homology Domain and Leucine-Rich Repeat Protein Phosphatase 2)-Mediated Dephosphorylation of AKT1 and eNOS (Endothelial NO Synthase) Promoting Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2020;40:1705–1721. doi: 10.1161/ATVBAHA.120.314172. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

