Integration of Nanometer-Range Label-to-Label Distances and Their Distributions into Modelling Approaches
- PMID: 36291578
- PMCID: PMC9599366
- DOI: 10.3390/biom12101369
Integration of Nanometer-Range Label-to-Label Distances and Their Distributions into Modelling Approaches
Abstract
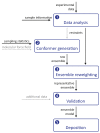
Labelling techniques such as electron paramagnetic resonance spectroscopy and single-molecule fluorescence resonance energy transfer, allow access to distances in the range of tens of angstroms, corresponding to the size of proteins and small to medium-sized protein complexes. Such measurements do not require long-range ordering and are therefore applicable to systems with partial disorder. Data from spin-label-based measurements can be processed into distance distributions that provide information about the extent of such disorder. Using such information in modelling presents several challenges, including a small number of restraints, the influence of the label itself on the measured distance and distribution width, and balancing the fitting quality of the long-range restraints with the fitting quality of other restraint subsets. Starting with general considerations about integrative and hybrid structural modelling, this review provides an overview of recent approaches to these problems and identifies where further progress is needed.
Keywords: EPR spectroscopy; FRET; double electron electron resonance; ensemble model; intrinsic disorder; molecular force fields; site-directed spin labelling; structural biology.
Conflict of interest statement
The authors declares no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

