Galectin-10 as a Potential Biomarker for Eosinophilic Diseases
- PMID: 36291593
- PMCID: PMC9599181
- DOI: 10.3390/biom12101385
Galectin-10 as a Potential Biomarker for Eosinophilic Diseases
Abstract
Galectin-10 is a member of the lectin family and one of the most abundant cytoplasmic proteins in human eosinophils. Except for some myeloid leukemia cells, basophils, and minor T cell populations, galectin-10 is exclusively present in eosinophils in the human body. Galectin-10 forms Charcot-Leyden crystals, which are observed in various eosinophilic diseases. Accumulating studies have indicated that galectin-10 acts as a new biomarker for disease activity, diagnosis, and treatment effectiveness in asthma, eosinophilic esophagitis, rhinitis, sinusitis, atopic dermatitis, and eosinophilic granulomatosis with polyangiitis. The extracellular release of galectin-10 is not mediated through conventional secretory processes (piecemeal degranulation or exocytosis), but rather by extracellular trap cell death (ETosis), which is an active cell death program. Eosinophils undergoing ETosis rapidly disintegrate their plasma membranes to release the majority of galectin-10. Therefore, elevated galectin-10 levels in serum and tissue suggest a high degree of eosinophil ETosis. To date, several studies have shown that galectin-10/Charcot-Leyden crystals are more than just markers for eosinophilic inflammation, but play functional roles in immunity. In this review, we focus on the close relationship between eosinophils and galectin-10, highlighting this protein as a potential new biomarker in eosinophilic diseases.
Keywords: Charcot–Leyden crystals; ETosis; eosinophil; eosinophil extracellular trap; galectin-10.
Conflict of interest statement
SU received grants and personal fees from AstraZeneca, personal fees from GlaxoSmithKline and Sanofi, and grants from Novartis and Maruho Co. Ltd.
Figures
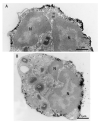
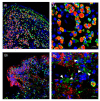

References
-
- Albers F.C., Licskai C., Chanez P., Bratton D.J., Bradford E.S., Yancey S.W., Kwon N., Quirce S. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir. Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

