Caenorhabditis elegans as a Model to Study Manganese-Induced Neurotoxicity
- PMID: 36291605
- PMCID: PMC9599542
- DOI: 10.3390/biom12101396
Caenorhabditis elegans as a Model to Study Manganese-Induced Neurotoxicity
Abstract
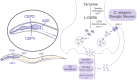
Caenorhabditis elegans (C. elegans) is a nematode present worldwide. The worm shows homology to mammalian systems and expresses approximately 40% of human disease-related genes. Since Dr. Sydney Brenner first proposed C. elegans as an advantageous experimental worm-model system for genetic approaches, increasing numbers of studies using C. elegans as a tool to investigate topics in several fields of biochemistry, neuroscience, pharmacology, and toxicology have been performed. In this regard, C. elegans has been used to characterize the molecular mechanisms and affected pathways caused by metals that lead to neurotoxicity, as well as the pathophysiological interrelationship between metal exposure and ongoing neurodegenerative disorders. Several toxic metals, such as lead, cadmium, and mercury, are recognized as important environmental contaminants, and their exposure is associated with toxic effects on the human body. Essential elements that are required to maintain cellular homeostasis and normal physiological functions may also be toxic when accumulated at higher concentrations. For instance, manganese (Mn) is a trace essential element that participates in numerous biological processes, such as enzymatic activities, energy metabolism, and maintenance of cell functions. However, Mn overexposure is associated with behavioral changes in C. elegans, which are consistent with the dopaminergic system being the primary target of Mn neurotoxicity. Caenorhabditis elegans has been shown to be an important tool that allows for studies on neuron morphology using fluorescent transgenic worms. Moreover, behavioral tests may be conducted using worms, and neurotransmitter determination and related gene expression are likely to change after Mn exposure. Likewise, mutant worms may be used to study molecular mechanisms in Mn toxicity, as well as the expression of proteins responsible for the biosynthesis, transport, storage, and uptake of dopamine. Furthermore, this review highlights some advantages and limitations of using the experimental model of C. elegans and provides guidance for potential future applications of this model in studies directed toward assessing for Mn neurotoxicity and related mechanisms.
Keywords: C. elegans; alternative animal models; manganese; neurodegeneration; neurotoxicology; trace elements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
High-throughput assessment of toxic effects of metal mixtures of cadmium(Cd), lead(Pb), and manganese(Mn) in nematode Caenorhabditis elegans.Chemosphere. 2019 Nov;234:232-241. doi: 10.1016/j.chemosphere.2019.05.271. Epub 2019 Jun 3. Chemosphere. 2019. PMID: 31220657
-
Toxicity interactions between manganese (Mn) and lead (Pb) or cadmium (Cd) in a model organism the nematode C. elegans.Environ Sci Pollut Res Int. 2018 Jun;25(16):15378-15389. doi: 10.1007/s11356-018-1752-5. Epub 2018 Mar 21. Environ Sci Pollut Res Int. 2018. PMID: 29564703
-
Metabolic effects of manganese in the nematode Caenorhabditis elegans through DAergic pathway and transcription factors activation.Neurotoxicology. 2018 Jul;67:65-72. doi: 10.1016/j.neuro.2018.04.008. Epub 2018 Apr 16. Neurotoxicology. 2018. PMID: 29673961
-
Altered manganese homeostasis: implications for BLI-3-dependent dopaminergic neurodegeneration and SKN-1 protection in C. elegans.J Trace Elem Med Biol. 2012 Jun;26(2-3):183-7. doi: 10.1016/j.jtemb.2012.03.011. Epub 2012 May 15. J Trace Elem Med Biol. 2012. PMID: 22591558 Review.
-
Neurodegeneration Induced by Metals in Caenorhabditis elegans.Adv Neurobiol. 2017;18:355-383. doi: 10.1007/978-3-319-60189-2_18. Adv Neurobiol. 2017. PMID: 28889277 Review.
Cited by
-
The Role of Oxidative Stress in Manganese Neurotoxicity: A Literature Review Focused on Contributions Made by Professor Michael Aschner.Biomolecules. 2023 Jul 28;13(8):1176. doi: 10.3390/biom13081176. Biomolecules. 2023. PMID: 37627240 Free PMC article. Review.
-
Caenorhabditis elegans as a suitable model to evaluate the toxicity of water from Rolante River, southern Brazil.Toxicol Res (Camb). 2023 Dec 18;13(1):tfad117. doi: 10.1093/toxres/tfad117. eCollection 2024 Feb. Toxicol Res (Camb). 2023. PMID: 38178995 Free PMC article.
-
Combined Restraint Stress and Metal Exposure Paradigms in Rats: Unravelling Behavioural and Neurochemical Perturbations.Mol Neurobiol. 2025 Apr;62(4):4355-4376. doi: 10.1007/s12035-024-04570-1. Epub 2024 Oct 24. Mol Neurobiol. 2025. PMID: 39443350
-
Simira cordifolia protects against metal induced-toxicity in Caenorhabditis elegans.Front Pharmacol. 2023 Nov 15;14:1235190. doi: 10.3389/fphar.2023.1235190. eCollection 2023. Front Pharmacol. 2023. PMID: 38035022 Free PMC article.
-
Glutamine supplementation reverses manganese neurotoxicity by eliciting the mitochondrial unfolded protein response.iScience. 2023 Jun 15;26(7):107136. doi: 10.1016/j.isci.2023.107136. eCollection 2023 Jul 21. iScience. 2023. PMID: 37408687 Free PMC article.
References
-
- Dingley S.D., Polyak E., Ostrovsky J., Srinivasan S., Lee I., Rosenfeld A.B., Tsukikawa M., Xiao R., Selak M.A., Coon J.J., et al. Mitochondrial DNA Variant in COX1 Subunit Significantly Alters Energy Metabolism of Geographically Divergent Wild Isolates in Caenorhabditis elegans. J. Mol. Biol. 2014;426:2199–2216. doi: 10.1016/j.jmb.2014.02.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

