Simulation of Calcium Dynamics in Realistic Three-Dimensional Domains
- PMID: 36291663
- PMCID: PMC9599163
- DOI: 10.3390/biom12101455
Simulation of Calcium Dynamics in Realistic Three-Dimensional Domains
Abstract
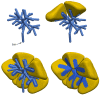
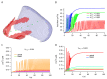
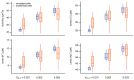
The cytosolic concentration of free calcium ions ([Ca2+]) is an important intracellular messenger in most cell types, and the spatial distribution of [Ca2+] is often critical. In a salivary gland acinar cell, a polarised epithelial cell, whose principal function is to transport water and thus secrete saliva, [Ca2+] controls the secretion of primary saliva, but increases in [Ca2+] are localised to the apical regions of the cell. Hence, any quantitative explanation of how [Ca2+] controls saliva secretion must take into careful account the spatial distribution of the various Ca2+ sources, Ca2+ sinks, and Ca2+-sensitive ion channels. Based on optical slices, we have previously constructed anatomically accurate three-dimensional models of seven salivary gland acinar cells, and thus shown that a model in which Ca2+ responses are confined to the apical regions of the cell is sufficient to provide a quantitative and predictive explanation of primary saliva secretion. However, reconstruction of such anatomically accurate cells is extremely time consuming and inefficient. Here, we present an alternative, mostly automated method of constructing three-dimensional cells that are approximately anatomically accurate and show that the new construction preserves the quantitative accuracy of the model.
Keywords: calcium dynamics; finite-element methods; saliva secretion; three-dimensional simulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

