Gene Delivery of Manf to Beta-Cells of the Pancreatic Islets Protects NOD Mice from Type 1 Diabetes Development
- PMID: 36291702
- PMCID: PMC9599570
- DOI: 10.3390/biom12101493
Gene Delivery of Manf to Beta-Cells of the Pancreatic Islets Protects NOD Mice from Type 1 Diabetes Development
Abstract
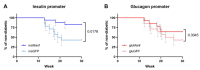
In type 1 diabetes, dysfunctional glucose regulation occurs due to the death of insulin-producing beta-cells in the pancreatic islets. Initiation of this process is caused by the inheritance of an adaptive immune system that is predisposed to responding to beta-cell antigens, most notably to insulin itself, coupled with unknown environmental insults priming the autoimmune reaction. While autoimmunity is a primary driver in beta-cell death, there is growing evidence that cellular stress participates in the loss of beta-cells. In the beta-cell fragility model, partial loss of islet mass requires compensatory upregulation of insulin production in the remaining islets, driving a cellular stress capable of triggering apoptosis in the remaining cells. The Glis3-Manf axis has been identified as being pivotal to the relative fragility or robustness of stressed islets, potentially operating in both type 1 and type 2 diabetes. Here, we have used an AAV-based gene delivery system to enhance the expression of the anti-apoptotic protein Manf in the beta-cells of NOD mice. Gene delivery substantially lowered the rate of diabetes development in treated mice. Manf-treated mice demonstrated minimal insulitis and superior preservation of insulin production. Our results demonstrating the therapeutic potential of Manf delivery to enhance beta-cell robustness and avert clinical diabetes.
Keywords: AAV; Manf; NOD mice; beta-cells; gene delivery; type 1 diabetes.
Conflict of interest statement
This work is the subject of active commercialization efforts by the Babraham Institute and VIB, which may result in financial return to authors. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Bonifacio E., Mathieu C., Nepom G.T., Ziegler A.G., Anhalt H., Haller M.J., Harrison L.C., Hebrok M., Kushner J.A., Norris J.M., et al. Rebranding asymptomatic type 1 diabetes: The case for autoimmune beta cell disorder as a pathological and diagnostic entity. Diabetologia. 2017;60:35–38. doi: 10.1007/s00125-016-4144-8. - DOI - PMC - PubMed
-
- Heianza Y., Arase Y., Fujihara K., Tsuji H., Saito K., Hsieh S.D., Kodama S., Shimano H., Yamada N., Hara S., et al. Screening for pre-diabetes to predict future diabetes using various cut-off points for HbA(1c) and impaired fasting glucose: The Toranomon Hospital Health Management Center Study 4 (TOPICS 4) Diabet. Med. 2012;29:e279–e285. doi: 10.1111/j.1464-5491.2012.03686.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

