Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3
- PMID: 36291716
- PMCID: PMC9599461
- DOI: 10.3390/biom12101507
Stereoselectivity in the Membrane Transport of Phenylethylamine Derivatives by Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3
Abstract
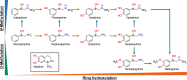
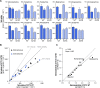
Stereoselectivity is well known and very pronounced in drug metabolism and receptor binding. However, much less is known about stereoselectivity in drug membrane transport. Here, we characterized the stereoselective cell uptake of chiral phenylethylamine derivatives by human monoamine transporters (NET, DAT, and SERT) and organic cation transporters (OCT1, OCT2, and OCT3). Stereoselectivity differed extensively between closely related transporters. High-affinity monoamine transporters (MATs) showed up to 2.4-fold stereoselective uptake of norepinephrine and epinephrine as well as of numerous analogs. While NET and DAT preferentially transported (S)-norepinephrine, SERT preferred the (R)-enantiomer. In contrast, NET and DAT showed higher transport for (R)-epinephrine and SERT for (S)-epinephrine. Generally, MAT stereoselectivity was lower than expected from their high affinity to several catecholamines and from the high stereoselectivity of some inhibitors used as antidepressants. Additionally, the OCTs differed strongly in their stereoselectivity. While OCT1 showed almost no stereoselective uptake, OCT2 was characterized by a roughly 2-fold preference for most (R)-enantiomers of the phenylethylamines. In contrast, OCT3 transported norphenylephrine and phenylephrine with 3.9-fold and 3.3-fold preference for their (R)-enantiomers, respectively, while the para-hydroxylated octopamine and synephrine showed no stereoselective OCT3 transport. Altogether, our data demonstrate that stereoselectivity is highly transporter-to-substrate specific and highly diverse even between homologous transporters.
Keywords: chiral HPLC; monoamine transporters; neurotransmitter transport; organic cation transporters; phenylethylamines; stereoselective drug transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Stereoselectivity in Cell Uptake by SLC22 Organic Cation Transporters 1, 2, and 3.J Med Chem. 2023 Dec 14;66(23):15990-16001. doi: 10.1021/acs.jmedchem.3c01436. Epub 2023 Dec 5. J Med Chem. 2023. PMID: 38052451 Free PMC article.
-
Stereoselective Inhibition of High- and Low-Affinity Organic Cation Transporters.Mol Pharm. 2023 Dec 4;20(12):6289-6300. doi: 10.1021/acs.molpharmaceut.3c00691. Epub 2023 Nov 14. Mol Pharm. 2023. PMID: 37962560 Free PMC article.
-
Stereoselective cell uptake of adrenergic agonists and antagonists by organic cation transporters.Biochem Pharmacol. 2020 Jan;171:113731. doi: 10.1016/j.bcp.2019.113731. Epub 2019 Nov 27. Biochem Pharmacol. 2020. PMID: 31783011
-
Organic Cation Transporters in Brain Catecholamine Homeostasis.Handb Exp Pharmacol. 2021;266:187-197. doi: 10.1007/164_2021_470. Handb Exp Pharmacol. 2021. PMID: 33987762 Review.
-
Stereoselectivity of chiral drug transport: a focus on enantiomer-transporter interaction.Drug Metab Rev. 2014 Aug;46(3):283-90. doi: 10.3109/03602532.2014.887094. Epub 2014 May 6. Drug Metab Rev. 2014. PMID: 24796860 Review.
Cited by
-
Permeability of Metformin across an In Vitro Blood-Brain Barrier Model during Normoxia and Oxygen-Glucose Deprivation Conditions: Role of Organic Cation Transporters (Octs).Pharmaceutics. 2023 Apr 28;15(5):1357. doi: 10.3390/pharmaceutics15051357. Pharmaceutics. 2023. PMID: 37242599 Free PMC article.
-
Stereoselectivity in Cell Uptake by SLC22 Organic Cation Transporters 1, 2, and 3.J Med Chem. 2023 Dec 14;66(23):15990-16001. doi: 10.1021/acs.jmedchem.3c01436. Epub 2023 Dec 5. J Med Chem. 2023. PMID: 38052451 Free PMC article.
-
Stereoselective Inhibition of High- and Low-Affinity Organic Cation Transporters.Mol Pharm. 2023 Dec 4;20(12):6289-6300. doi: 10.1021/acs.molpharmaceut.3c00691. Epub 2023 Nov 14. Mol Pharm. 2023. PMID: 37962560 Free PMC article.
References
-
- Hernandez M.A., Rathinavelu A. Basic Pharmacology: Understanding Drug Actions and Reactions. Routledge; London, UK: 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

