The Effect of Positive Charge Distribution on the Cryoprotective Activity of Dehydrins
- PMID: 36291719
- PMCID: PMC9599493
- DOI: 10.3390/biom12101510
The Effect of Positive Charge Distribution on the Cryoprotective Activity of Dehydrins
Abstract
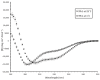
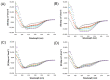
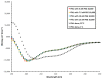
Dehydrins are intrinsically disordered proteins expressed ubiquitously throughout the plant kingdom in response to desiccation. Dehydrins have been found to have a cryoprotective effect on lactate dehydrogenase (LDH) in vitro, which is in large part influenced by their hydrodynamic radius rather than the order of the amino acids within the sequence (alternatively, this may be a sequence specific effect). However, it seems that a different mechanism may underpin the cryoprotection that they confer to the cold-labile yeast frataxin homolog-1 (Yfh1). Circular dichroism spectroscopy (CD) was used to assess the degree of helicity of Yfh1 at 1 °C, both alone and in the presence of several dehydrin constructs. Three constructs were compared to the wild type: YSK2-K→R (lysine residues substituted with arginine), YSK2-Neutral (locally neutralized charge), and YSK2-SpaceK (evenly distributed positive charge). The results show that sequence rearrangements and minor substitutions have little impact on the ability of the dehydrin to preserve LDH activity. However, when the positive charge of the dehydrin is locally neutralized or evenly distributed, the dehydrin becomes less efficient at promoting structure in Yfh1 at low temperatures. This suggests that a stabilizing, charge-based interaction occurs between dehydrins and Yfh1. Dehydrins are intrinsically disordered proteins, expressed by certain organisms to improve desiccation tolerance. These proteins are thought to serve many cellular roles, such as the stabilization of membranes, DNA, and proteins. However, the molecular mechanisms underlying the function of dehydrins are not well understood. Here, we examine the importance of positive charges in dehydrin sequences by making substitutions and comparing their effects in the cryoprotection of two different proteins.
Keywords: charge; circular dichroism; cryoprotection; dehydrins; intrinsically disordered proteins; lactate dehydrogenase; sequence order; yeast frataxin homolog 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Sequence composition versus sequence order in the cryoprotective function of an intrinsically disordered stress-response protein.Protein Sci. 2019 Aug;28(8):1448-1459. doi: 10.1002/pro.3648. Epub 2019 May 29. Protein Sci. 2019. PMID: 31102309 Free PMC article.
-
Cryoprotective activity of Arabidopsis KS-type dehydrin depends on the hydrophobic amino acids of two active segments.Arch Biochem Biophys. 2020 Sep 30;691:108510. doi: 10.1016/j.abb.2020.108510. Epub 2020 Jul 28. Arch Biochem Biophys. 2020. PMID: 32735864
-
The Disordered Dehydrin and Its Role in Plant Protection: A Biochemical Perspective.Biomolecules. 2022 Feb 11;12(2):294. doi: 10.3390/biom12020294. Biomolecules. 2022. PMID: 35204794 Free PMC article. Review.
-
Structural and Functional Insights into the Cryoprotection of Membranes by the Intrinsically Disordered Dehydrins.J Biol Chem. 2015 Nov 6;290(45):26900-26913. doi: 10.1074/jbc.M115.678219. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370084 Free PMC article.
-
Disorder and function: a review of the dehydrin protein family.Front Plant Sci. 2014 Oct 31;5:576. doi: 10.3389/fpls.2014.00576. eCollection 2014. Front Plant Sci. 2014. PMID: 25400646 Free PMC article. Review.
Cited by
-
Comparative Analysis of Dehydrins from Woody Plant Species.Biomolecules. 2024 Feb 20;14(3):250. doi: 10.3390/biom14030250. Biomolecules. 2024. PMID: 38540671 Free PMC article.
-
Plant dehydrins and dehydrin-like proteins: characterization and participation in abiotic stress response.Front Plant Sci. 2023 Jul 6;14:1213188. doi: 10.3389/fpls.2023.1213188. eCollection 2023. Front Plant Sci. 2023. PMID: 37484455 Free PMC article. Review.
References
-
- Cheng Z., Targolli J., Huang X., Wu R. Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice (Oryza sativa L.) Mol. Breed. 2002;10:71–82. doi: 10.1023/A:1020329401191. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

