The Tumor Microenvironment of Medulloblastoma: An Intricate Multicellular Network with Therapeutic Potential
- PMID: 36291792
- PMCID: PMC9599673
- DOI: 10.3390/cancers14205009
The Tumor Microenvironment of Medulloblastoma: An Intricate Multicellular Network with Therapeutic Potential
Abstract
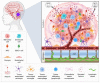
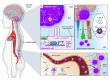
Medulloblastoma (MB) is a heterogeneous disease in which survival is highly affected by the underlying subgroup-specific characteristics. Although the current treatment modalities have increased the overall survival rates of MB up to 70-80%, MB remains a major cause of cancer-related mortality among children. This indicates that novel therapeutic approaches against MB are needed. New promising treatment options comprise the targeting of cells and components of the tumor microenvironment (TME). The TME of MB consists of an intricate multicellular network of tumor cells, progenitor cells, astrocytes, neurons, supporting stromal cells, microglia, immune cells, extracellular matrix components, and vasculature systems. In this review, we will discuss all the different components of the MB TME and their role in MB initiation, progression, metastasis, and relapse. Additionally, we briefly introduce the effect that age plays on the TME of brain malignancies and discuss the MB subgroup-specific differences in TME components and how all of these variations could affect the progression of MB. Finally, we highlight the TME-directed treatments, in which we will focus on therapies that are being evaluated in clinical trials.
Keywords: age-associated differences; brain tumor vasculature; extracellular matrix; immune cells; leptomeningeal dissemination; medulloblastoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Roles and interactions of tumor microenvironment components in medulloblastoma with implications for novel therapeutics.Genes Chromosomes Cancer. 2024 Apr;63(4):e23233. doi: 10.1002/gcc.23233. Genes Chromosomes Cancer. 2024. PMID: 38607297 Review.
-
Decoding the Roles of Astrocytes and Hedgehog Signaling in Medulloblastoma.Curr Oncol. 2021 Aug 11;28(4):3058-3070. doi: 10.3390/curroncol28040267. Curr Oncol. 2021. PMID: 34436033 Free PMC article. Review.
-
Characterization of immune microenvironment associated with medulloblastoma metastasis based on explainable machine learning.Pediatr Investig. 2025 Feb 14;9(1):59-69. doi: 10.1002/ped4.12471. eCollection 2025 Mar. Pediatr Investig. 2025. PMID: 40241883 Free PMC article.
-
Medulloblastoma Spatial Transcriptomics Reveals Tumor Microenvironment Heterogeneity with High-Density Progenitor Cell Regions Correlating with High-Risk Disease.bioRxiv [Preprint]. 2024 Jun 28:2024.06.25.600684. doi: 10.1101/2024.06.25.600684. bioRxiv. 2024. PMID: 38979174 Free PMC article. Preprint.
-
Complement C3a activates astrocytes to promote medulloblastoma progression through TNF-α.J Neuroinflammation. 2022 Jun 20;19(1):159. doi: 10.1186/s12974-022-02516-9. J Neuroinflammation. 2022. PMID: 35725556 Free PMC article.
Cited by
-
Inhibition of metastatic brain cancer in Sonic Hedgehog medulloblastoma using caged nitric oxide albumin nanoparticles.Front Oncol. 2023 May 3;13:1129533. doi: 10.3389/fonc.2023.1129533. eCollection 2023. Front Oncol. 2023. PMID: 37213306 Free PMC article.
-
The Role of CyberKnife Stereotactic Radiosurgery in Recurrent Cranial Medulloblastomas across Pediatric and Adult Populations.J Clin Med. 2024 Jun 19;13(12):3592. doi: 10.3390/jcm13123592. J Clin Med. 2024. PMID: 38930121 Free PMC article.
-
Dual targeting of CD155/TIGIT and PD-L1/PD-1 immune checkpoints potentiates NK cell-mediated cytotoxicity in medulloblastoma.Neurooncol Adv. 2025 May 18;7(1):vdaf099. doi: 10.1093/noajnl/vdaf099. eCollection 2025 Jan-Dec. Neurooncol Adv. 2025. PMID: 40703802 Free PMC article.
-
Non-cellular immunotherapies in pediatric central nervous system tumors.Front Immunol. 2023 Oct 11;14:1242911. doi: 10.3389/fimmu.2023.1242911. eCollection 2023. Front Immunol. 2023. PMID: 37885882 Free PMC article. Review.
-
Medulloblastoma: biology and immunotherapy.Front Immunol. 2025 Jul 3;16:1602930. doi: 10.3389/fimmu.2025.1602930. eCollection 2025. Front Immunol. 2025. PMID: 40677711 Free PMC article. Review.
References
-
- Riemondy K.A., Venkataraman S., Willard N., Nellan A., Sanford B., Griesinger A.M., Amani V., Mitra S., Hankinson T.C., Handler M.H., et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro. Oncol. 2022;24:273–286. doi: 10.1093/neuonc/noab135. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

