Atypical Macropinocytosis Contributes to Malignant Progression: A Review of Recent Evidence in Endometrioid Endometrial Cancer Cells
- PMID: 36291839
- PMCID: PMC9599675
- DOI: 10.3390/cancers14205056
Atypical Macropinocytosis Contributes to Malignant Progression: A Review of Recent Evidence in Endometrioid Endometrial Cancer Cells
Abstract
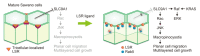
Macropinocytosis is an essential mechanism for the non-specific uptake of extracellular fluids and solutes. In recent years, additional functions have been identified in macropinocytosis, such as the intracellular introduction pathway of drugs, bacterial and viral infection pathways, and nutritional supplement pathway of cancer cells. However, little is known about the changes in cell function after macropinocytosis. Recently, it has been reported that macropinocytosis is essential for endometrial cancer cells to initiate malignant progression in a dormant state. Macropinocytosis is formed by a temporary split of adjacent bicellular junctions of epithelial sheets, rather than from the apical surface or basal membrane, as a result of the transient reduction of tight junction homeostasis. This novel type of macropinocytosis has been suggested to be associated with the malignant pathology of endometriosis and endometrioid endometrial carcinoma. This review outlines the induction of malignant progression of endometrial cancer cells by macropinocytosis based on a new mechanism and the potential preventive mechanism of its malignant progression.
Keywords: LSR; dormancy; endometrioid-endometrial carcinoma; endometriosis; macropinocytosis; tight junctions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Roles of Tricellular Tight Junction Protein Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor (LSR) in Endometriosis and Endometrioid-Endometrial Carcinoma.Cancers (Basel). 2021 Dec 17;13(24):6341. doi: 10.3390/cancers13246341. Cancers (Basel). 2021. PMID: 34944960 Free PMC article. Review.
-
The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells.Oncotarget. 2016 May 10;7(19):27735-52. doi: 10.18632/oncotarget.8408. Oncotarget. 2016. PMID: 27036040 Free PMC article.
-
Translocation of LSR from tricellular corners causes macropinocytosis at cell-cell interface as a trigger for breaking out of contact inhibition.FASEB J. 2021 Sep;35(9):e21742. doi: 10.1096/fj.202100299R. FASEB J. 2021. PMID: 34403506
-
Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer.Oncol Lett. 2017 Dec;14(6):6776-6782. doi: 10.3892/ol.2017.7038. Epub 2017 Sep 22. Oncol Lett. 2017. PMID: 29151917 Free PMC article.
-
The Role and Therapeutic Potential of Macropinocytosis in Cancer.Front Pharmacol. 2022 Aug 15;13:919819. doi: 10.3389/fphar.2022.919819. eCollection 2022. Front Pharmacol. 2022. PMID: 36046825 Free PMC article. Review.
Cited by
-
Extracellular Microvesicles Modified with Arginine-Rich Peptides for Active Macropinocytosis Induction and Delivery of Therapeutic Molecules.ACS Appl Mater Interfaces. 2024 Apr 10;16(14):17069-17079. doi: 10.1021/acsami.3c14592. Epub 2024 Apr 2. ACS Appl Mater Interfaces. 2024. PMID: 38563247 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

