Dialysis as a Novel Adjuvant Treatment for Malignant Cancers
- PMID: 36291840
- PMCID: PMC9600214
- DOI: 10.3390/cancers14205054
Dialysis as a Novel Adjuvant Treatment for Malignant Cancers
Abstract
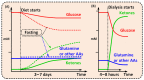
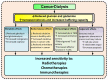
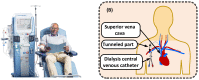
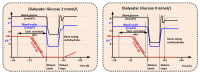
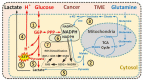
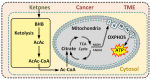
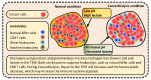
Cancer metabolism is characterized by an increased utilization of fermentable fuels, such as glucose and glutamine, which support cancer cell survival by increasing resistance to both oxidative stress and the inherent immune system in humans. Dialysis has the power to shift the patient from a state dependent on glucose and glutamine to a ketogenic condition (KC) combined with low glutamine levels-thereby forcing ATP production through the Krebs cycle. By the force of dialysis, the cancer cells will be deprived of their preferred fermentable fuels, disrupting major metabolic pathways important for the ability of the cancer cells to survive. Dialysis has the potential to reduce glucose levels below physiological levels, concurrently increase blood ketone body levels and reduce glutamine levels, which may further reinforce the impact of the KC. Importantly, ketones also induce epigenetic changes imposed by histone deacetylates (HDAC) activity (Class I and Class IIa) known to play an important role in cancer metabolism. Thus, dialysis could be an impactful and safe adjuvant treatment, sensitizing cancer cells to traditional cancer treatments (TCTs), potentially making these significantly more efficient.
Keywords: HDAC; cancer; chemotherapies; dialysis; immunotherapies; ketone bodies; radiotherapies; redox balance.
Conflict of interest statement
A.C. (Ana Carneiro), S.K., H.A. and K.P. have received consultancy grants from Baxter International Inc. for expert support and participation in the project. C.Ö. has received consultancy grants from Baxter International Inc. for in silico and in vivo studies. Excepting that, the authors declare no conflict of interest.
Figures


References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

